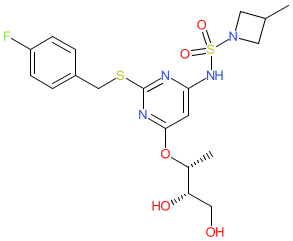
|
Synonyms: AZD-4721 | AZD4721 | compound 4 [WO2019055509A1] | RIST4721
Compound class:
Synthetic organic
Comment: Vimnerixin (AZD4721/RIST4721 ) is an orally bioavailable CXC chemokine receptor type 2 antagonist [1]. It has minimal activity on CXCR1, so has little effect on neutrophil activation.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Vimnerixin (RIST4721, AZD4721) has progressed to clinical evaluation in chronic inflammatory conditions. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01962935 | Study to Investigate Safety, Tolerability and Effect of Multiple Dosing With AZD 4721 and/or With AZD 5069 | Phase 1 Interventional | AstraZeneca | ||
| NCT05348681 | A Study to Evaluate RIST4721 in Hidradenitis Suppurativa (HS) | Phase 2 Interventional | Aristea Therapeutics, Inc. | ||
| NCT05448391 | A Study to Evaluate RIST4721 in Familial Mediterranean Fever (FMF) | Phase 2 Interventional | Aristea Therapeutics, Inc. | ||
| NCT03988335 | A Study to Evaluate RIST4721 in Palmoplantar Pustulosis (PPP) | Phase 2 Interventional | Aristea Therapeutics, Inc. | 1 | |