|
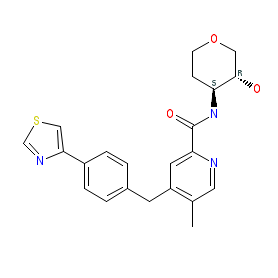
Synonyms: PF06767832
Compound class:
Synthetic organic
Comment: PF-06767832 (compound 38) is an orally bioavailable, positive allosteric modulator (PAM) selective for the M1 muscarinic acetylcholine receptor (CHRM1) [2]. It is one of the compounds claimed in patent WO2016009297 [1]. M1 receptor PAMs are being designed for their potential to provide novel therapies for the treatment of M1-mediated diseases and disorders such as Alzheimer's disease.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| In relation to Alzheimer's disease, stimulation of the M1 receptor has been shown to increase formation of the neuroprotective soluble amyloid precursor protein alpha (sAPPa; a proteolyte of APP cleavage by α-secretase), and in so doing, prevents the formation of the Αβ peptide [3]. However, despite not activating the M2 or M3 receptors, in vivo testing in dogs reveals that positive allosteric modulation of M1 causes the gastrointestinal and cardiovascular side-effects (cholinergic liabilities) , that were originally attributed to M2 and M3 receptor activation [2]. |