|
Synonyms: Example 12 [US9468635B2] | KBP-5074 | KBP5074
Compound class:
Synthetic organic
Comment: Ocedurenone (KBP-5074) is a selective and potent non-steroidal mineralocorticoid receptor (MR) antagonist that was developed by KBP Biosciences for management of uncontrolled/resistant hypertension in advanced chronic kidney disease [3]. The non-steroidal MR antagonist class of drugs includes finerenone and esaxerenone which are already in clinical use. These agents typically demonstrate a lower risk of inducing hyperkalemia [2,5] and with reduced hormonal side effects compared to steroidal MR antagonists such as spironolactone and eplerenone.
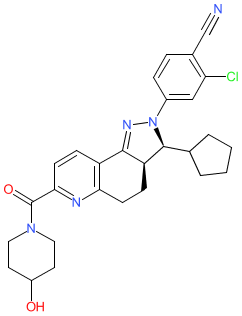
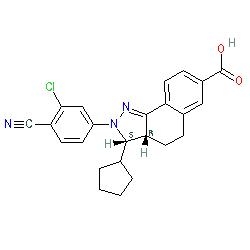
The chemical structure for KBP-5074 that's depicted here, was disclosed in [3], and is the (3S,3aR) enantiomer, and this matches the structure submitted for the INN ocedurenone. This chemical structure is one of those claimed in KBP Biosciences' patent US9468635B2 (Example 12 therein) [4]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||