|
Compound class:
Synthetic organic
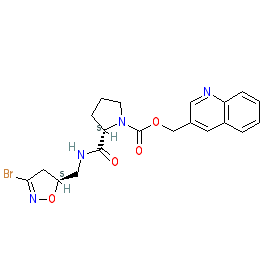
Comment: ERW1041E is a transglutaminase inhibitor [3]. We drew the structure as depicted in Chrobok et al. (2018) [1], with the indicated stereochemistry (but note that the IUPAC they provide is for the flat structure that is represented by PubChem CID 11619556).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Chrobok NL, Bol JGJM, Jongenelen CA, Brevé JJP, El Alaoui S, Wilhelmus MMM, Drukarch B, van Dam AM. (2018)
Characterization of Transglutaminase 2 activity inhibitors in monocytes in vitro and their effect in a mouse model for multiple sclerosis. PLoS ONE, 13 (4): e0196433 eCollection 2018. DOI: 10.1371/journal.pone.0196433 [PMID:29689097] |
|
2. Dafik L, Albertelli M, Stamnaes J, Sollid LM, Khosla C. (2012)
Activation and inhibition of transglutaminase 2 in mice. PLoS ONE, 7 (2): e30642. [PMID:22319575] |
|
3. Watts RE, Siegel M, Khosla C. (2006)
Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J Med Chem, 49 (25): 7493-501. [PMID:17149878] |