|
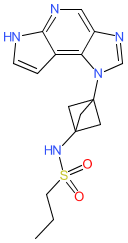
Synonyms: compound 1 [US20220009927]
Compound class:
Synthetic organic
Comment: The chemical structure for zemprocitinib was obtained from proposed INN list 130 (Feb. 2024), in which the compound is described as a Janus kinase inhibitor with anti-inflammatory potential. There is a structure match for zemprocitinib in patent US20220009927 (Lynk Pharmaceuticals) [1]. We suspect that zemprocitinib may be Lynk's lead JAK1 inhibitor LNK01001, but this association has not been formally disclosed.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| LNK01001 is a therapeutic candidate for the treatment of autoinflammatory conditions such as rheumatoid arthritis and atopic dermatitis. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06277245 | A Phase 3 Study of LNK01001 Capsule in Subjects With Moderate to Severe Atopic Dermatitis. | Phase 3 Interventional | Lynk Pharmaceuticals Co., Ltd | ||
| NCT06276998 | A Phase 3 Study of LNK01001 Capsule in Moderately to Severely Active Rheumatoid Arthritis | Phase 3 Interventional | Lynk Pharmaceuticals Co., Ltd | ||