|
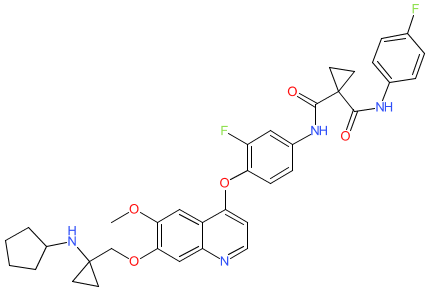
Synonyms: Example 6 [US20120123126] [1]
Compound class:
Synthetic organic
Comment: The chemical structure for vilzemetkib was obtained from proposed INN list 130 (Feb. 2024), in which the compound is described as an hepatocyte growth factor receptor (c-Met) inhibitor with proposed antineoplastic action. The structure is claimed in patents including some filed by Advenchen laboratories and the Chia Tai Tianqing Pharmaceutical Group. It is likely that vilzemetkib is Advenchen's lead oral c-Met inhibitor AL2846.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| AL2846 is an active clinical candidate in a range of trials in patients with advanced solid tumours. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05745363 | Clinical Study of AL2846 Capsule in the Treatment of Differentiated Thyroid Cancer | Phase 1/Phase 2 Interventional | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | ||
| NCT05815862 | Clinical Study of AL2846 Capsules in the Treatment of Advanced Lung Tumor and Advanced Ovarian Cancer | Phase 2 Interventional | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | ||
| NCT05011019 | A Clinical Trial to Evaluate the Safety and Efficacy of AL2846 Capsules in Chinese Patients With Type I Neurofibromatosis | Phase 1/Phase 2 Interventional | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | ||
| NCT05922345 | Evaluation of the Efficacy and Safety of AL2846 Capsule Combined With TQB2450 Injection Compared to Docetaxel Injection in Advanced Non-small Cell Lung Cancer Patients Who Have Failed With Immunotherapy. | Phase 3 Interventional | Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | ||