|
Synonyms: ABDNAZ [2] | RRx-001 | RRx001
Compound class:
Synthetic organic
Comment: RRx-001 (EpicentRx) was originally developed as an anti-tumour agent [3]; it promotes apoptosis, and has radio- , chemo- and immuno-sensitising actions in cancer models. It also provides cytoprotective effects in normal tissues, which has led to the proposal of RRx-001 as a supportive care drug in patients undergoing cytotoxic cancer therapy. RRx-001 was identified using phenotypic screening, so its molecular mechanism of action was initially unclear. Several mechanisms have subsequently been identified, including NLRP3 inflammasome inhibition [1,4] and CD47/SIRP-α immune checkpoint inhibition. RRx-001 covalently targets the cysteine 409 residue in NLRP3's NACHT domain and blocks NLRP3/NEK7 interaction and assembly of the inflammasome complex. This ultimately inhibits release of active pro-inflammatory cytokines (IL-1β and IL-18).
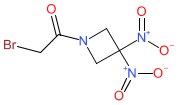
RRx-001's chemical structure is a match for the INN nibrozetone (proposed INN list 130 of Feb. 2024). |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| RRx-001 is a clinical candidate for the treament of advanced solid tumours. There are plans to evaluate its potential to block inflammasome activation [1,4] as a route to target neuroinflammation as a driver of Parkinson's pathology [5]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05566041 | A Phase 3, Controlled, Open-label, Global Randomized Study of RRx-001 With a Platinum Doublet or a Platinum Doublet in Small Cell Lung Cancer | Phase 3 Interventional | EpicentRx, Inc. | ||
| NCT03699956 | RRx-001 Sequentially With a Platinum Doublet or a Platinum Doublet in Third-Line or Beyond in Patients With Small Cell Lung Cancer | Phase 3 Interventional | EpicentRx, Inc. | ||