|
Synonyms: ZLN 005 | ZLN-005
Compound class:
Synthetic organic
Comment: ZLN005 acts as a transcriptional activator of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; PPARGC1A) [6]. Activation of the AMP-activated protein kinase pathway is implicated in the induction of PGC-1α expression. ZLN005 appears to produce cytoprotective/neuroprotective actions by enhancing mitochondrial fatty acid oxidation, respiration and biogenesis in a range of cell types and pathologies [1,3-5,7], and attenuating oxidative stress and necroptosis.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
IUPAC Name  |
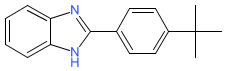
| 2-(4-tert-butylphenyl)-1H-benzimidazole |
Synonyms  |
| ZLN 005 | ZLN-005 |
Database Links  |
|
| ChEMBL Ligand | CHEMBL4303368 |
| GtoPdb PubChem SID | 491300037 |
| PubChem CID | 899323 |
| Search Google for chemical match using the InChIKey | LQUNNCQSFFKSSK-UHFFFAOYSA-N |
| Search Google for chemicals with the same backbone | LQUNNCQSFFKSSK |
| UniChem Compound Search for chemical match using the InChIKey | LQUNNCQSFFKSSK-UHFFFAOYSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | LQUNNCQSFFKSSK-UHFFFAOYSA-N |