GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
MET proto-oncogene, receptor tyrosine kinase
 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb
Target id: 1815
Nomenclature: MET proto-oncogene, receptor tyrosine kinase
Abbreviated Name: MET
Family: Type X RTKs: HGF (hepatocyte growth factor) receptor family
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Enzyme Reaction
- Natural/Endogenous Ligands
- Activators
- Inhibitors
- Antibodies
- Other Binding Ligands
- Large-scale Ligand Screening Data
- Immunopharmacology Comments
- Clinically-Relevant Mutations and Pathophysiology
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 1390 | 7q31 | MET | MET proto-oncogene, receptor tyrosine kinase | |
| Mouse | 1 | 1379 | 6 7.83 cM | Met | met proto-oncogene, receptor tyrosine kinase | |
| Rat | 1 | 1382 | 4q21 | Met | MET proto-oncogene, receptor tyrosine kinase | |
Database Links  |
|
| Alphafold | P08581 (Hs), P16056 (Mm), P97523 (Rn) |
| BRENDA | 2.7.10.1 |
| CATH/Gene3D | 2.60.40.10, 2.130.10.10 |
| ChEMBL Target | CHEMBL3717 (Hs), CHEMBL5585 (Mm) |
| DrugBank Target | P08581 (Hs) |
| Ensembl Gene | ENSG00000105976 (Hs), ENSMUSG00000009376 (Mm), ENSRNOG00000052745 (Rn) |
| Entrez Gene | 4233 (Hs), 17295 (Mm) |
| Human Protein Atlas | ENSG00000105976 (Hs) |
| KEGG Enzyme | 2.7.10.1 |
| KEGG Gene | hsa:4233 (Hs), mmu:17295 (Mm) |
| OMIM | 164860 (Hs) |
| Orphanet | ORPHA123201 (Hs) |
| Pharos | P08581 (Hs) |
| RefSeq Nucleotide | NM_000245 (Hs), NM_008591 (Mm), NM_031517 (Rn) |
| RefSeq Protein | NP_000236 (Hs), NP_032617 (Mm), NP_113705 (Rn) |
| SynPHARM |
81931 (in complex with AM7) 78753 (in complex with crizotinib) 79023 (in complex with crizotinib) 81202 (in complex with foretinib) 79050 (in complex with foretinib) 84917 (in complex with merestinib) 84918 (in complex with merestinib) 81064 (in complex with SGX-523) 81205 (in complex with SGX-523) 83121 (in complex with tepotinib) |
| UniProtKB | P08581 (Hs), P16056 (Mm), P97523 (Rn) |
| Wikipedia | MET (Hs) |
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
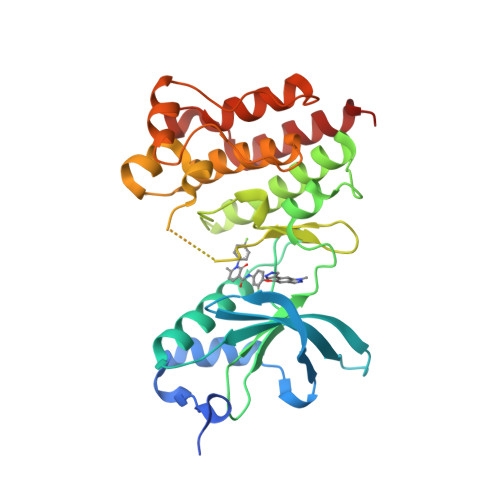
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Natural/Endogenous Ligands  |
| hepatocyte growth factor {Sp: Human} |
Download all structure-activity data for this target as a CSV file 
| Activators | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tepotinib was shown to have an IC50 of <1nM in an in vitro study with recombinant human MET [20]. Tanespimycin is reported as a femtomolar inhibitor of MET-induced urokinase plasminogen activation (uPA) pathway [48]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antibodies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Binding Ligands | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 19,54 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: MET | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: MET(M1250T) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: MET(Y1235D) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 2,23 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: Met/c-MET | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The leucine-rich repeat (LRR)-containing protein InlB of Listeria monocytogenes is reported to mediate bacterial entry in to non-phagocytic host cells by binding to the MET proto-oncogene, receptor tyrosine kinase (MET, or hepatocyte growth factor receptor, HGFR) [5]. Tanespimycin (a potent inhibitor of MET [48], in addition to Hsp90) has reported efficacy against intracellular infection of non-phagocytic cells by L. monocytogenes [43]. |
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
References
1. Ai J, Chen Y, Peng X, Ji Y, Xi Y, Shen Y, Yang X, Su Y, Sun Y, Gao Y et al.. (2018) Preclinical Evaluation of SCC244 (Glumetinib), a Novel, Potent, and Highly Selective Inhibitor of c-Met in MET-dependent Cancer Models. Mol Cancer Ther, 17 (4): 751-762. [PMID:29237805]
2. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
3. Bannen LC, Bui M, Jiang F, Tso K, Wang Y, Xu W. (2019) Compounds for the treatment of kinase-dependent disorders. Patent number: WO2019148044A1. Assignee: Exelixis, Inc.. Priority date: 26/01/2018. Publication date: 01/08/2019.
4. Bellon SF, Kaplan-Lefko P, Yang Y, Zhang Y, Moriguchi J, Rex K, Johnson CW, Rose PE, Long AM, O'Connor AB et al.. (2008) c-Met inhibitors with novel binding mode show activity against several hereditary papillary renal cell carcinoma-related mutations. J Biol Chem, 283 (5): 2675-83. [PMID:18055465]
5. Bierne H, Cossart P. (2002) InlB, a surface protein of Listeria monocytogenes that behaves as an invasin and a growth factor. J Cell Sci, 115 (Pt 17): 3357-67. [PMID:12154067]
6. Bladt F, Faden B, Friese-Hamim M, Knuehl C, Wilm C, Fittschen C, Grädler U, Meyring M, Dorsch D, Jaehrling F et al.. (2013) EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c-Met inhibitors. Clin Cancer Res, 19 (11): 2941-51. [PMID:23553846]
7. Bode CM, Boezio AA, Albrecht BK, Bellon SF, Berry L, Broome MA, Choquette D, Dussault I, Lewis RT, Lin MH et al.. (2012) Discovery and optimization of a potent and selective triazolopyridinone series of c-Met inhibitors. Bioorg Med Chem Lett, 22 (12): 4089-93. [PMID:22595176]
8. Boezio AA, Berry L, Albrecht BK, Bauer D, Bellon SF, Bode C, Chen A, Choquette D, Dussault I, Fang M et al.. (2009) Discovery and optimization of potent and selective triazolopyridazine series of c-Met inhibitors. Bioorg Med Chem Lett, 19 (22): 6307-12. [PMID:19819693]
9. Bromberg JS, Weir MR, Gaber AO, Yamin MA, Goldberg ID, Mayne TJ, Cal W, Cooper M. (2021) Renal Function Improvement Following ANG-3777 Treatment in Patients at High Risk for Delayed Graft Function After Kidney Transplantation. Transplantation, 105 (2): 443-450. [PMID:32265417]
10. Buchanan SG, Hendle J, Lee PS, Smith CR, Bounaud PY, Jessen KA, Tang CM, Huser NH, Felce JD, Froning KJ et al.. (2009) SGX523 is an exquisitely selective, ATP-competitive inhibitor of the MET receptor tyrosine kinase with antitumor activity in vivo. Mol Cancer Ther, 8 (12): 3181-90. [PMID:19934279]
11. Burggraaf J, Kamerling IM, Gordon PB, Schrier L, de Kam ML, Kales AJ, Bendiksen R, Indrevoll B, Bjerke RM, Moestue SA et al.. (2015) Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med, 21 (8): 955-61. [PMID:26168295]
12. Chen GP. (2012) Compounds As c-Met Kinase Inhibitors. Patent number: US20120123126A1. Assignee: Chen GP. Priority date: 08/09/2011. Publication date: 17/05/2012.
13. Cho SY, Lee BH, Jung H, Yun CS, Ha JD, Kim HR, Chae CH, Lee JH, Seo HW, Oh KS. (2013) Design and synthesis of novel 3-(benzo[d]oxazol-2-yl)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine derivatives as selective G-protein-coupled receptor kinase-2 and -5 inhibitors. Bioorg Med Chem Lett, 23 (24): 6711-6. [PMID:24210504]
14. Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R et al.. (2003) A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res, 63 (21): 7345-55. [PMID:14612533]
15. Claridge S, Raeppel F, Granger MC, Bernstein N, Saavedra O, Zhan L, Llewellyn D, Wahhab A, Deziel R, Rahil J et al.. (2008) Discovery of a novel and potent series of thieno[3,2-b]pyridine-based inhibitors of c-Met and VEGFR2 tyrosine kinases. Bioorg Med Chem Lett, 18 (9): 2793-8. [PMID:18434145]
16. Cui JJ, Rogers EW, Ung J, Whitten J, Zhai D, Deng W, Zhang X, Huang Z, Liu J, Zhang H. (2019) Macrocyclic compounds and uses thereof. Patent number: WO2019023417A1. Assignee: Tp Therapeutics, Inc.. Priority date: 28/07/2017. Publication date: 31/01/2019.
17. Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, Pairish M, Jia L, Meng J, Funk L, Botrous I et al.. (2011) Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem, 54 (18): 6342-63. [PMID:21812414]
18. Davies J, Liu L, Lu J, Vaillancourt PE, Wortinger MA, Zeng W. c-Met antibodies. Patent number: US8217148. Assignee: Eli Lilly And Company. Priority date: 21/11/2008. Publication date: 10/07/2012.
19. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
20. Dorsch D, Schadt O, Stieber F, Meyring M, Grädler U, Bladt F, Friese-Hamim M, Knühl C, Pehl U, Blaukat A. (2015) Identification and optimization of pyridazinones as potent and selective c-Met kinase inhibitors. Bioorg Med Chem Lett, 25 (7): 1597-602. [PMID:25736998]
21. Flynn DL, Kaufman MD, Smith BD. (2016) Inhibition of tumor cell interactions with the microenvironment resulting in a reduction in tumor growth and disease progression. Patent number: WO2016061231. Assignee: Deciphera Pharmaceuticals, Llc. Priority date: 14/10/2014. Publication date: 21/04/2016.
22. Fujita H, Miyadera K, Kato M, Fujioka Y, Ochiiwa H, Huang J, Ito K, Aoyagi Y, Takenaka T, Suzuki T et al.. (2013) The novel VEGF receptor/MET-targeted kinase inhibitor TAS-115 has marked in vivo antitumor properties and a favorable tolerability profile. Mol Cancer Ther, 12 (12): 2685-96. [PMID:24140932]
23. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
24. Hart AC, Abell L, Guo J, Mertzman ME, Padmanabha R, Macor JE, Chaudhry C, Lu H, O'Malley K, Shaw PJ et al.. (2019) Identification of RIPK3 Type II Inhibitors Using High-Throughput Mechanistic Studies in Hit Triage. ACS Med Chem Lett, Article ASAP. DOI: 10.1021/acsmedchemlett.9b00065
25. Hu H, Mu Q, Bao Z, Chen Y, Liu Y, Chen J, Wang K, Wang Z, Nam Y, Jiang B et al.. (2018) Mutational Landscape of Secondary Glioblastoma Guides MET-Targeted Trial in Brain Tumor. Cell, 175 (6): 1665-1678.e18. [PMID:30343896]
26. Huang A, Schwall R, Yansura D. (2005) Monovalent antibody fragments useful as therapeutics. Patent number: US20050227324 A1. Assignee: Genentech, Inc.. Priority date: 19/12/2003. Publication date: 13/10/2005.
27. Jarantow SW, Bushey BS, Pardinas JR, Boakye K, Lacy ER, Sanders R, Sepulveda MA, Moores SL, Chiu ML. (2015) Impact of Cell-surface Antigen Expression on Target Engagement and Function of an Epidermal Growth Factor Receptor × c-MET Bispecific Antibody. J Biol Chem, 290 (41): 24689-704. [PMID:26260789]
28. Jeong H-j, Ha JD, Cho S-y, Kim H-r, Kwangho L, Jungok L, Choi CW, Park J. (2015) Novel triazolopyrazine derivative and use thereof. Patent number: WO2015046653A1. Assignee: Korea Research Institute of Chemical Technology, Handok Co., Ltd.. Priority date: 30/09/2013. Publication date: 02/04/2015.
29. Jia H, Dai G, Weng J, Zhang Z, Wang Q, Zhou F, Jiao L, Cui Y, Ren Y, Fan S et al.. (2014) Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J Med Chem, 57 (18): 7577-89. [PMID:25148209]
30. Kinoshita K, Asoh K, Furuichi N, Ito T, Kawada H, Hara S, Ohwada J, Miyagi T, Kobayashi T, Takanashi K et al.. (2012) Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem, 20 (3): 1271-80. [PMID:22225917]
31. Kong-Beltran M, Wickramasinghe DM. (2009) Methods and compositions for modulating hyperstabilized c-met. Patent number: US7615529 B2. Assignee: Genentech, Inc.. Priority date: 05/01/2016. Publication date: 10/11/2009.
32. Li S, Huang Q, Liu Y, Zhang X, Liu S, He C, Gong P. (2013) Design, synthesis and antitumour activity of bisquinoline derivatives connected by 4-oxy-3-fluoroaniline moiety. Eur J Med Chem, 64: 62-73. [PMID:23644189]
33. Liu X, Wang Q, Yang G, Marando C, Koblish HK, Hall LM, Fridman JS, Behshad E, Wynn R, Li Y et al.. (2011) A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res, 17 (22): 7127-38. [PMID:21918175]
34. Lovly CM, Heuckmann JM, de Stanchina E, Chen H, Thomas RK, Liang C, Pao W. (2011) Insights into ALK-driven cancers revealed through development of novel ALK tyrosine kinase inhibitors. Cancer Res, 71 (14): 4920-31. [PMID:21613408]
35. Lu T, Alexander R, Connors RW, Cummings MD, Galemmo RA, Hufnagel HR, Johnson DL, Khalil E, Leonard KA, Markotan TP et al.. (2007) Triazolopyridazines as tyrosine kinase modulators. Patent number: WO2007075567A1. Assignee: Janssen Pharmaceutica, N.V.. Priority date: 21/12/2005. Publication date: 05/07/2007.
36. Milkiewicz KL, Aimone LD, Albom MS, Angeles TS, Chang H, Grobelny JV, Husten J, Losardo C, Miknyoczki S, Murthy S et al.. (2011) Improvement in oral bioavailability of 2,4-diaminopyrimidine c-Met inhibitors by incorporation of a 3-amidobenzazepin-2-one group. Bioorg Med Chem, 19 (21): 6274-84. [PMID:21967808]
37. Munshi N, Jeay S, Li Y, Chen CR, France DS, Ashwell MA, Hill J, Moussa MM, Leggett DS, Li CJ. (2010) ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther, 9 (6): 1544-53. [PMID:20484018]
38. Musumeci F, Radi M, Brullo C, Schenone S. (2012) Vascular endothelial growth factor (VEGF) receptors: drugs and new inhibitors. J Med Chem, 55 (24): 10797-822. [PMID:23098265]
39. Nakagawa T, Tohyama O, Yamaguchi A, Matsushima T, Takahashi K, Funasaka S, Shirotori S, Asada M, Obaishi H. (2010) E7050: a dual c-Met and VEGFR-2 tyrosine kinase inhibitor promotes tumor regression and prolongs survival in mouse xenograft models. Cancer Sci, 101 (1): 210-5. [PMID:19832844]
40. Ouyang XS, Grandinetti KB, Boren M, Chakravorty S, Chopade S, Jiang P, Kanouni T, Koudriakova T, Makwana O, Pack SK et al.. (2025) Discovery of KIN-8741, a Highly Selective Type IIb c-Met Kinase Inhibitor with Broad Mutation Coverage and Quality Drug-Like Properties for the Treatment of Cancer. J Med Chem, 68 (11): 10648-10662. [PMID:40459881]
41. Pan BS, Chan GK, Chenard M, Chi A, Davis LJ, Deshmukh SV, Gibbs JB, Gil S, Hang G, Hatch H et al.. (2010) MK-2461, a novel multitargeted kinase inhibitor, preferentially inhibits the activated c-Met receptor. Cancer Res, 70 (4): 1524-33. [PMID:20145145]
42. Patwardhan PP, Ivy KS, Musi E, de Stanchina E, Schwartz GK. (2016) Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma. Oncotarget, 7 (4): 4093-109. [PMID:26675259]
43. Puthiyakunnon S, He X, Boddu S, Huang SH, Cao H. (2017) C-Met Inhibitors are Potential Novel Therapeutic Agents Against Listeria monocytogenes Infection Through Blocking the Bacteria Entry into Nonphagocytic Cells. Curr Top Med Chem, 17 (3): 278-289. [PMID:27572078]
44. Qi B, Yang Y, Gong G, He H, Yue X, Xu X, Hu Y, Li J, Chen T, Wan X et al.. (2019) Discovery of N1-(4-((7-(3-(4-ethylpiperazin-1-yl)propoxy)-6-methoxyquinolin-4-yl)oxy)-3,5-difluorophenyl)-N3-(2-(2,6-difluorophenyl)-4-oxothiazolidin-3-yl)urea as a multi-tyrosine kinase inhibitor for drug-sensitive and drug-resistant cancers treatment. Eur J Med Chem, 163: 10-27. [PMID:30503936]
45. Schiering N, Knapp S, Marconi M, Flocco MM, Cui J, Perego R, Rusconi L, Cristiani C. (2003) Crystal structure of the tyrosine kinase domain of the hepatocyte growth factor receptor c-Met and its complex with the microbial alkaloid K-252a. Proc Natl Acad Sci USA, 100 (22): 12654-9. [PMID:14559966]
46. Schlapbach A, Feifel R, Hawtin S, Heng R, Koch G, Moebitz H, Revesz L, Scheufler C, Velcicky J, Waelchli R et al.. (2008) Pyrrolo-pyrimidones: a novel class of MK2 inhibitors with potent cellular activity. Bioorg Med Chem Lett, 18 (23): 6142-6. [PMID:18945615]
47. Schroeder GM, An Y, Cai ZW, Chen XT, Clark C, Cornelius LA, Dai J, Gullo-Brown J, Gupta A, Henley B et al.. (2009) Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem, 52 (5): 1251-4. [PMID:19260711]
48. Shen Y, Xie Q, Norberg M, Sausville E, Vande Woude G, Wenkert D. (2005) Geldanamycin derivative inhibition of HGF/SF-mediated Met tyrosine kinase receptor-dependent urokinase-plasminogen activation. Bioorg Med Chem, 13 (16): 4960-71. [PMID:15978816]
49. Slavish PJ, Price JE, Jiang Q, Cui X, Morris SW, Webb TR. (2011) Synthesis of an aryloxy oxo pyrimidinone library that displays ALK-selective inhibition. Bioorg Med Chem Lett, 21 (15): 4592-6. [PMID:21708465]
50. Wang C, Li J, Qu L, Tang X, Song X, Yang F, Chen X, Lin Q, Lin W, Zhou Y et al.. (2022) Discovery of D6808, a Highly Selective and Potent Macrocyclic c-Met Inhibitor for Gastric Cancer Harboring MET Gene Alteration Treatment. J Med Chem, 65 (22): 15140-15164. [PMID:36355693]
51. Wang J, Anderson MG, Oleksijew A, Vaidya KS, Boghaert ER, Tucker L, Zhang Q, Han EK, Palma JP, Naumovski L et al.. (2017) ABBV-399, a c-Met Antibody-Drug Conjugate that Targets Both MET-Amplified and c-Met-Overexpressing Tumors, Irrespective of MET Pathway Dependence. Clin Cancer Res, 23 (4): 992-1000. [PMID:27573171]
52. Wang X, Le P, Liang C, Chan J, Kiewlich D, Miller T, Harris D, Sun L, Rice A, Vasile S et al.. (2003) Potent and selective inhibitors of the Met [hepatocyte growth factor/scatter factor (HGF/SF) receptor] tyrosine kinase block HGF/SF-induced tumor cell growth and invasion. Mol Cancer Ther, 2 (11): 1085-92. [PMID:14617781]
53. Weinberg LR, Albom MS, Angeles TS, Husten J, Lisko JG, McHugh RJ, Milkiewicz KL, Murthy S, Ott GR, Theroff JP et al.. (2011) Fused bicyclic derivatives of 2,4-diaminopyrimidine as c-Met inhibitors. Bioorg Med Chem Lett, 21 (1): 164-7. [PMID:21123062]
54. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
55. Xi N. (2015) Substituted quinoline compounds and methods of use. Patent number: US9133162B2. Assignee: Sunshine Lake Pharma Co Ltd, Calitor Sciences LLC. Priority date: 28/02/2011. Publication date: 15/09/2015.
56. Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B et al.. (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther, 10 (12): 2298-308. [PMID:21926191]
57. Yan SB, Peek VL, Ajamie R, Buchanan SG, Graff JR, Heidler SA, Hui YH, Huss KL, Konicek BW, Manro JR et al.. (2013) LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest New Drugs, 31 (4): 833-44. [PMID:23275061]
58. You WK, Sennino B, Williamson CW, Falcón B, Hashizume H, Yao LC, Aftab DT, McDonald DM. (2011) VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res, 71 (14): 4758-68. [PMID:21613405]
59. Zhao J, Fang L, Zhang X, Liang Y, Gou S. (2016) Synthesis and biological evaluation of new [1,2,4]triazolo[4,3-a]pyridine derivatives as potential c-Met inhibitors. Bioorg Med Chem, 24 (16): 3483-93. [PMID:27288183]













