GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
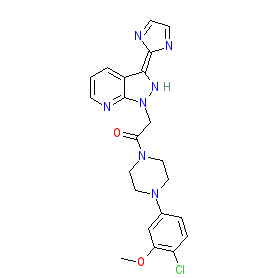
Synonyms: CCX 354 | CCX-354 | CCX-354-C
Compound class:
Synthetic organic
Comment: CCX354 is an orally active CCR1 antagonist that was originally developed by Chemocentryx for anti-inflammatory/immunomodulatory potential. The chemical structure is claimed as Example 16 in patent WO2008147815A1 [1]. The structure in the patent resolves to PubChem CID 25016615, which is a tautomeric representation of the claimed structure.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| CCX354 (as code CCX-354-C) completed Phase 2 clinical evaluation in rheumatoid arthritis patients in which it appeared to be safe and well tolerated, and showed some evidence of clinical efficacy [2], but development was not progressed further (see NCT01242917). |
Mechanism Of Action and Pharmacodynamic Effects  |
| CCX354 downregulates migration of monocytes and macrophages to synovial tissue, by antagonising the effects of CCR1 chemokines such as CCL3 (MIP-1α) and CCL5 (RANTES). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01242917 | A Study to Evaluate the Safety and Efficacy of CCX354-C in Subjects With Rheumatoid Arthritis Partially Responsive to Methotrexate Therapy | Phase 2 Interventional | ChemoCentryx | ||