|
Synonyms: Seconal®
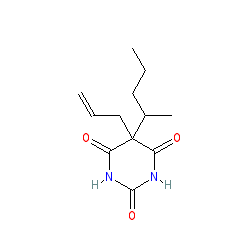
secobarbital is an approved drug (FDA (1950))
Compound class:
Synthetic organic
Comment: The approved drug is a racemic mixture of enantiomers; R-secobarbital and S-secobarbital. We show the non-stereo structure to represent the mixture.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: secobarbital |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Secobarbital is used short-term to treat insomnia, as a pre-operative sedative, or as an anaesthetic for short surgical, diagnostic, or therapeutic procedures. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Secobarbital is a positive allosteric modulator of GABAA channels, enhancing the inhibitory effects of GABA in the CNS. The drug binds to a barbituate allosteric site [1], which is distinct from the benzodiazepine allosteric site, and from the GABA binding site. Barbiturate modulation prolongs the duration of bursts of chloride channel opening [1] and this is responsible for the anxiolytic, anticonvulsant, amnesic, sedative, hypnotic, euphoriant, and muscle relaxant properties of the drug. |
External links  |
|
For extended ADME data see the following: Drugs.com |