|
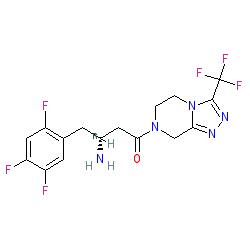
Synonyms: Januvia® | MK-0431 | Zituvio®
sitagliptin is an approved drug (FDA (2006), EMA (2007))
Compound class:
Synthetic organic
Comment: Sitagliptin is a dipeptidyl peptidase 4 (DPP4) inhibitor. Marketed formulations may contain sitagliptin phosphate monohydrate (PubChem CID 11591741).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: sitagliptin |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Davis JA, Singh S, Sethi S, Roy S, Mittra S, Rayasam G, Bansal V, Sattigeri J, Ray A. (2010)
Nature of action of Sitagliptin, the dipeptidyl peptidase-IV inhibitor in diabetic animals. Indian J Pharmacol, 42 (4): 229-33. [PMID:20927248] |
|
2. Kieffer TJ, McIntosh CH, Pederson RA. (1995)
Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology, 136 (8): 3585-96. [PMID:7628397] |
|
3. Tewary S, Lucas ES, Fujihara R, Kimani PK, Polanco A, Brighton PJ, Muter J, Fishwick KJ, Da Costa MJMD, Ewington LJ et al.. (2020)
Impact of sitagliptin on endometrial mesenchymal stem-like progenitor cells: A randomised, double-blind placebo-controlled feasibility trial. EBioMedicine, 51: 102597. DOI: 10.1016/j.ebiom.2019.102597 [PMID:31928963] |