GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
P2Y receptors: Introduction
General
P2Y receptors are one of three families of extracellular receptors for purine and pyrimidine nucleotides involved in purinergic signalling [11]. Adenosine (previously known as P1) receptors and the P2X receptors are classified by separate NC-IUPHAR Subcommittees. The division of P2 nucleotide receptors into two families - a P2X ion channel family and a P2Y G protein-coupled receptor family - first proposed by Abbracchio and Burnstock [2], has been generally adopted with growing numbers of receptors recognised since that time [74].
P2Y receptors are a multigene family
The first genes for P2Y receptor subtypes were cloned in 1993 with the isolation of cDNAs from chick brain [86] and a murine neuroblastoma (NG108-15) cell line [54]. These two cDNAs encode structurally related metabotropic proteins that are activated either by adenine nucleotides (chick P2Y1 receptor) or by adenine and uracil nucleotides (murine P2Y2 receptor). The structural similarities between the chick P2Y1 and mouse P2Y2 receptor proteins provided the requisite tools for a wave of homology screening. Thereafter, the numbers of species orthologues of P2Y1 and P2Y2 receptors, and of novel P2Y-like sequences, grew at a rapid rate, with the corresponding need to periodically review classification of the family.
Current classification
The mammalian P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 receptors, and the non-mammalian receptors chick p2y3, Xenopus p2y8 and turkey p2y, are known to be functional nucleotide receptors [1,3,12,14,17,38,48,74]. Only the mammalian receptors are formally classified by NC-IUPHAR. There is much uncertainty about the human p2y5 receptor and, for the moment, it should still be considered an orphan receptor. Also, p2y9 and p2y10 are orphan receptors (i.e. no known agonists) [42,74] while the receptor protein originally called p2y7 is actually a leukotriene B4 (LTB4) receptor [89]. The long-sought platelet ADP receptor [originally called P2T [33], and also called P2T [40], P2TAC [44,51], P2YAC receptor [78], P2Ycyc [35], P2YADP [28], P2Y?[13] has been cloned in 2001 (P2Y12). P2Y13 and P2Y14 receptors have been characterized during a systematic study of orphan receptors and are now formally recognised [1,17]. In addition to these cloned P2Y receptors, another endogenous P2Y receptor is recognised: the dinucleotide receptor[73] [alternatively called P2D [71], P2YApnA [28], P4 [72]. There may exist additional yet-unidentified P2Y receptors [1,3].
Structural features of of nucleotide receptors
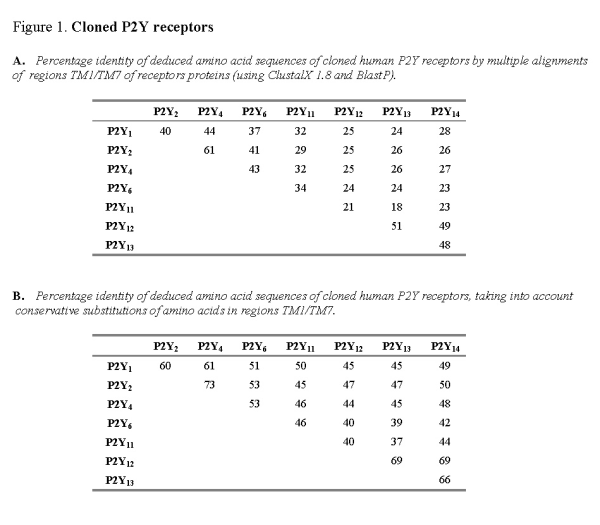
It is believed that the transmembrane regions TMI-VII of metabotropic proteins form the nucleotide binding pocket of P2Y receptors [41]. The extracellular N-terminus of the protein is not critically important for agonist binding, whereas the intracellular C-terminus is believed to influence signal transduction. The structural relatedness of nucleotide receptors can be compared by multiple alignments of the deduced amino acid sequence for TMI-VII for each receptor protein (Figure 1A). Alignment of human receptor sequences (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) shows this subfamily of nucleotide receptors to be approximately 21-61% identical. Where conservative substitutions are taken into account (i.e., where amino acids have been exchanged for other amino acids of similar structure and charge), the degree of relatedness amongst human nucleotide receptors is much higher (37-69% identical) (Figure 1B). It might be expected that the nucleotide binding pocket created by the region TM1-VII should show a high degree of structural relatedness.
Species homology
The degree of structural relatedness between P2Y receptor proteins has been viewed as a means to identify species homologues, particularly where non-mammalian and human P2Y receptors have been compared[12]. Thus, it has been proposed that chick p2y3 is the species homologue of h P2Y6 on the basis of sequence identity (66%), whilst Xenopus p2y8 and turkey p2y have been viewed as species homologues of human P2Y4 (61% and 62% identical, respectively). However, there are no clear guidelines to say what level of sequence identity is categorically predictive. For example, chick P2Y1 and turkey P2Y1 are much more closely related to human P2Y1 (both 89% identical) and, therefore, levels of homology in the 60--80% range might not be so meaningful. Furthermore, some closely related species homologues (human P2Y4 and rat P2Y4; 90% identical) actually have different pharmacological profiles, so that phenotype alone is not predictive of relatedness. The P2Y2 and P2Y4 proteins (62% identical), encoded by different genes (on chromosomes 11 and X, respectively), are structurally related and phenotypically different. Thus, it is a difficult process to successfully predict which P2Y receptor proteins are true species homologues.
Nucleotide binding sites
Regarding the molecular nature of the nucleotide binding pocket, useful information has been made available from SAR studies of human P2Y1 and mouse P2Y2 receptors [23,41,43]. Analysis of agonist activity at mutant P2Y1 receptors revealed that TM6 and TM7 lie close to the adenine ring, TM3 and TM6 lie close to the ribose moiety, and key residues in TM3, TM5, TM6 and TM7 lie near the triphosphate chain[41]. Positively charged residues in TM3 and TM7 may interact with the α-phosphate, and TM5 residues with the γ-phosphate of nucleotidic agonists [43]. Two disulphide bridges in the extracellular domain were identified as essential (perhaps to stabilise the receptor structure), whilst positively charged lysine and arginine residues in the second and third extracellular loop (EL) are essential for high agonist potency [41]. Site-directed mutagenesis of mouse P2Y2 receptors also reveals the importance of positively charged residues in TM6 (H-X-X-R/K) and TM7 (Y-Q/K-X-X-R) in nucleotide triphosphate binding [23], consistent with findings on the human P2Y1 receptor [41]. In P2Y12, P2Y13 and P2Y14 receptors, the (Y-Q/K-X-X-R) motif in TM7 is substituted by the highly conserved motif K-E-X-X-L which might affect ligand binding characteristics [1]. More recently, for these receptors, one additional K residue in EL2 has been suggested to be particularly important for nucleotide binding [21].
P2Y receptor signal transduction
In heterologous expression systems, recombinant P2Y1, P2Y2, P2Y4, P2Y6 receptors couple to G proteins (mainly Gq) which, in turn, activate intracellular signalling cascades - mainly the phospholipase Cβ (PLCβ) isoform to mobilise intracellular Ca2+ ions[12]. Some recombinant P2Y receptors (e.g. P2Y11) also affect intracellular cAMP levels [9,12,18] while P2Y12, P2Y13, P2Y14 mainly couple to Gi/o proteins and inhibit cAMP formation. Hydrophobicity plots of deduced amino acid sequences for functional P2Y receptors indicate that seven segments (TMI-VII) of the receptor protein cross the plasmalemma, while the N-terminus of the protein resides on the extracellular side and C-terminus on the intracellular side of the cell [54,86]. Each transmembrane region is believed to form an α-helix and, accordingly, P2Y receptors are sometimes described as heptahelical proteins. Therefore, P2Y receptors are part of the Rhodopsin superfamily [81].
Recombinant P2Y receptors affect a narrow range of signalling pathways (mainly PLCβ and adenylate cyclase, AC) in heterologous expression systems, whereas endogenous metabotropic P2 receptors affect a much wider range of intracellular signalling cascades (PLCβ, PLD, PLA2, AC, MEK/MAP kinase system, Rho-dependent kinase). The reasons for the narrow selectivity of signal transduction by recombinant P2Y receptors have not been explained but, in part, may be related to a limited or insufficient availability of the relevant effector targets in the expression systems so far utilised.
Correspondence of native and cloned P2Y receptors
A major challenge is understanding the correspondence between native and recombinant nucleotide-activated G protein-coupled receptors [48]. For a number of reasons, it has proved exceedingly problematic to associate particular recombinant P2Y receptors with metabotropic P2 receptors found in mammalian tissues. It is often the case that several P2Y receptors are co-expressed in any one tissue or cell type. With the exception of P2Y1 and P2Y12, there is a paucity of selective agonists and antagonists to clearly distinguish one P2Y receptor from another. Furthermore, pharmacological analyses of metabotropic P2 receptors have often been hampered by the presence of ecto-enzymes (surface enzymes), which i) remove one or more phosphate groups attached to the ribose moiety of the nucleotide to change the nature of the agonist (e.g. ATP into ADP); and ii) exchange phosphates from an exogenously applied nucleotide to an endogenous nucleotide released by the host cell thereby generating another agonist (e.g. generating UTP from ATP, via locally released UDP). In addition to those G protein-coupled receptors activated principally or exclusively by dinucleotides (e.g. ApnAs), there may be additional P2Y receptors on the human genome, as suggested by the presence of yet-uncharacterized "P2Y-like" receptors [1,3]. A further problem involves the disparities in the types of signalling cascades utilised by native and recombinant P2 receptors. Lastly, the notion has been raised that P2Y receptors may in theory dimerize, to generate homodimeric P2Y receptors with significantly different pharmacological profiles from the related monomeric receptor[25], or heterodimeric P2Y receptors with novel phenotypes [58]. By analogy, the β2-adrenoceptor has been reported to form homodimeric receptors [34], whereas opioid receptors are capable of forming heterodimeric complexes [45].
Pharmacological properties of P2Y receptors
Mammalian Receptors
The P2Y1 receptor is activated by adenine nucleotides and not by uracil nucleotides. Avian P2Y1 receptors are activated by ATP and ADP [26,86], whereas mammalian P2Y1 receptors are potently activated mainly by ADP and related diphosphate analogues[77]. Studies of the human P2Y1 receptor indicate that ATP and related triphosphates can act in some cases as receptor antagonists [36,56] whilst, in other cases, ATP and related triphosphates can act as partial agonists when receptor reserve is high and as antagonists when receptor reserve is low [68]. Additionally, ATP and related triphosphates may act as full agonists when P2Y1 receptor number is in excess and, theoretically, when homodimeric P2Y1 receptors are present [25]. Homologues of P2Y1 receptors are blocked by PPADS, a substance originally proposed to be selective for P2X receptors, and also are blocked by suramin. However, adenosine bisphosphate derivatives are much more potent antagonists (MRS2179 and MRS2279) [8,63-64]. A viable P2Y1 knockout mouse model has been developed [24,55].
The P2Y2 receptor is activated by both adenine and uracil nucleotides, and by their triphosphates but not diphosphates [54,67]. Species homologues of the P2Y2 receptor are not known to possess different pharmacological profiles. Other uracil dinucleotides, such as INS 365 (Up4U) and INS 37217 (Up4dC), also potently activate the P2Y2 receptor [79,88]. The P2Y2 receptor is blocked by suramin but is insensitive to PPADS.
The human P2Y4 receptor is activated by uridine nucleotidic triphosphates (not diphosphates) and is considered to be a pyrimidinoceptor [20,66]. ATP is reported to be an antagonist at the human P2Y4 receptor [47]. However, the rat P2Y4 receptor, like the P2Y2 receptor, is activated by both ATP and UTP [7,46-47,84]. The rat P2Y4 receptor is blocked by Reactive blue 2 but is insensitive to suramin and PPADS [7]. This suramin-insensitivity distinguishes the rat P2Y4 from the rat P2Y2 receptor which is blocked by suramin.
P2Y6 receptor is activated by uracil 5'-diphosphate derivates, while MRS2567 and MRS2578 act as antagonists both at the human and rat P2Y6 receptor [57]. RT-PCR and Northern Blotting studies show an high expression of this receptor in spleen and placenta, followed by kidney, adipose, bone, lung, heart, brain and skeletal muscle [19,61].
The human P2Y11 receptor is activated by adenine nucleotides, principally ATP and weakly by ADP, but not by uracil nucleotides [18,58]. This ATP receptor is unique in promoting the elevation of both IP3 and cAMP levels with agonist activation in heterologous expression systems. A similar behaviour has been demonstrated for turkey p2y receptor, which is activated by all naturally occurring nucleoside triphosphates, resulting in activation of PLCβ and inhibition of adenylate cyclase [9] (see also below). Thus, the ability of human P2Y11 and turkey p2y to modulate cAMP levels distinguishes these proteins from other recombinant P2Y receptors.
The long-sought platelet receptor for ADP is the P2Y12 receptor, which is activated by adenine nucleotidic disphosphates (2-MeSADP>>ADP>ADPβS) [32]. The rat [38] and mouse [27] receptors have also been identified and characterized. P2Y12 receptor is primarily coupled to Gi/o protein and is highly expressed in platelets where it plays a role in amplification of platelet activation and aggregation. The active metabolite of clopidogrel and ticlopidine act as potent P2Y12 antagonists and are efficient antithrombotic drugs [76]. The AR-C compounds, including AR-C69931MX, which are all ATP analogues [39] act as potent direct competitive P2Y12M antagonists. The P2Y12 receptor has also been shown to be expressed in subregions of the brain [38]. Glial cells [5,30,75], brain capillary endothelial cells [80] and, recently reported, smooth muscle cells [87] and chromaffin cells [22], express P2Y12 receptors, although the precise role of this receptor subtype in these cells is still under study. P2Y12 knockout mice [4,27] display the phenotype of clopidogrel-treated animals i.e. prolonged bleeding time and inhibition of platelet aggregation to ADP.
The human [17,90], mouse [90] and rat [31] receptors have been identified and characterized. ADP is the naturally agonist of the P2Y13 receptor, but the relative potencies of ADP and 2-MeSADP differed according to the assays used. AR-C69931MX is also an antagonist of the human and rat P2Y13 receptors [31,59]. Ap4A and 2-MeSAMP are two other P2Y13 antagonists [59]. P2Y13 receptor is primarily coupled to a Go/i protein [17,59]. P2Y13 mRNA is highly expressed in the spleen followed by placenta, liver, bone marrow, lung and various brain regions [90]. A significant expression in human monocytes or bone marrow, but not in human platelets [82,90] has also been found.
The human P2Y14 receptor (previously known as GPR105 or UDP-glucose receptor) has been cloned by Chambers and coworkers and the complete sequence of the rat (VTR-15-20, [16,29]) and mouse orthologs [29] have been also described. The P2Y14 receptor is activated by UDP-glucose as well as UDP-galactose, UDP-glucuronic acid and UDP-N-acetylglucosamine but not by uracil or adenine nucleotides [14]. At present, no selective antagonists are available, although it has to be underlined that the currently available P2 receptor antagonists have not been tested on this receptor. P2Y14 receptor couples to the Gi/o family of G proteins. P2Y14 mRNA is widely distributed in the human body, with moderate to high levels observed in placenta, adipose tissue, stomach, intestine, selected brain regions (e.g. c. striatum, cerebellum, caudate nucleus, hippocampus, hypothalamus), spleen, lung, heart, bone marrow and thymus. RT-PCR revealed also expression in brain glial cells [5,30] and prominent expression in neutrophils, lymphocytes and megakaryocytic cells [14,62].
Non-mammalian Receptors
The current NC-IUPHAR policy is to classify receptors from mammalian systems, but this does not fully recognise the importance of data gleaned from non-mammalian receptors. The first cloned P2Y receptor, P2Y1, was cloned from chick brain and, only through homology screening, was the P2Y1 receptor extended to mammalian receptors. Other non-mammalian receptors have since been cloned from Xenopus laevis (p2y8) and chick (p2y3 and p2y5). In most cases, the discovery of non-mammalian receptors has led to the discovery of mammalian counterparts. Although no mammalian orthologue has been investigated for p2y8, this receptor is clearly of the P2Y family and sufficiently novel to be of pharmacological interest, as well as appearing to subserve an important role in neurogenesis in frog [6] (see also below). Inclusion of this and other non-mammalian receptors would provide a fuller account for investigators interested in all aspects of the P2Y receptor family.
The chick p2y3 receptor activated by adenine and uracil nucleotides, (mostly by their diphosphates and much less so by triphosphates) likely represents the species ortholog of hP2Y6 [83]. The pharmacological profile of the chick p2y3 receptor when expressed in Jurkat cells (UDP-preferring) is different from that seen when the receptor is expressed in Xenopus oocytes (ADP-preferring). The chick p2y3 receptor is blocked by suramin in both expression systems, and by Reactive blue 2 in the oocyte. The turkey p2y3 homologue, expressed in human astrocytoma cells, is UDP-preferring [52]. Thus, the chick p2y3 and turkey p2y3 phenotypes observed in mammalian expression systems show a reasonable similarity to the pharmacological profile of rat and human P2Y6 receptors which, themselves, are considered to be UDP receptors [15,19]. This similarity in phenotype provides a measure of support for the notion that avian p2y3 and mammalian P2Y6 receptors are species homologues.
Avian and mammalian homologues of the so-called p2y5 receptor have been cloned [37,53,85], but there is scant evidence to show these proteins are true nucleotide receptors. Radiolabelled nucleotides bind to chick p2y5 receptors expressed in COS-7 cells [85], whereas the turkey homologue failed to alter IP3 and cAMP levels when expressed in human astrocytoma cells [53]. Further work is required to fully assess mammalian p2y5 homologues as nucleotide receptors.
As mentioned above, an avian receptor, turkey p2y, is activated by adenine and uracil nucleotides and shows a marked preference for triphosphates [10]. The turkey p2y receptor is activated by all naturally occurring nucleoside triphosphates (ATP, CTP, GTP, ITP, UTP, XTP) resulting in the activation of PLCβ and inhibition of adenylate cyclase [9].
A novel non-mammalian P2Y receptor, p2y8, was cloned from Xenopus laevis embryos and shown to be activated by all naturally occurring nucleoside triphosphates (ATP, CTP, GTP, ITP and UTP) but not by inorganic polyphosphates [6]. Expression of Xenopus p2y8 in the amphibian neural plate is temporally related with neurogenesis, indicating a possible role for P2Y receptors in early neuronal development. The P2Y1 receptor is also involved in chick embryonic development, although mainly in non-neural tissues [60]. P2Y1 receptors have also been implicated in the development of neonatal rat salivary glands [69], whilst P2Y2 receptors appear to be involved in mouse T cell differentiation [50]. ATP acting via endogenous P2Y receptors has profound trophic actions on neurones and astrocytic cells in culture [65], and the role of nucleotides in cell development, differentiation and growth represents an important area for future research.
Receptor nomenclature
P2Y is used for functional mammalian receptor proteins and functional receptor proteins from non-mammalian species. The lower-case, p2y, is used for non-mammalian receptors without a mammalian homologue. The subscript numbers (1-n) following P2Y or p2y sequentially list proteins in their chronological order of cDNA cloning. An example of this nomenclature is c P2Y1, which is a functional chick P2Y receptor with functional mammalian homologues (h P2Y1, b P2Y1, m P2Y1, r P2Y1) and also the first cDNA cloned. Another example is x p2y8, a functional Xenopus laevis P2Y receptor with no known mammalian homologue and the eighth cDNA cloned. A final example is h p2y9, a human P2Y-like protein that does not function as a nucleotide receptor and, chronologically, was cloned after x p2y8.
Non-mammalian receptors
The current NC-IUPHAR policy is to classify receptors from mammalian systems, but this does not fully recognise the importance of data gleaned from non-mammalian receptors. The first cloned P2Y receptor, P2Y1, was cloned from chick brain and, only through homology screening, was the P2Y1 receptor extended to mammalian receptors. Other non-mammalian receptors have since been cloned from Xenopus laevis (p2y8) and chick (p2y3 and p2y5).
In most cases, the discovery of non-mammalian receptors has led to the discovery of mammalian counterparts. Although no mammalian orthologue has been investigated for p2y8, this receptor is clearly of the P2Y family and sufficiently novel to be of pharmacological interest, as well as appearing to subserve an important role in neurogenesis in frog [6]. Inclusion of this and other non-mammalian receptors would provide a fuller account for investigators interested in all aspects of the P2Y receptor family.
P2 purinergic receptors and inflammation
ATP acting via P2 receptors (P2Y and P2X family members) serves as an essential mediator of functional responses in human eosinophils [49], responses which may be implicated in the pathogenesis of various forms of airway inflammation [70].
References
1. Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA et al.. (2003) Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci, 24 (2): 52-5. [PMID:12559763]
2. Abbracchio MP, Burnstock G. (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors?. Pharmacol Ther, 64 (3): 445-75. [PMID:7724657]
3. Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA et al.. (2005) The recently deorphanized GPR80 (GPR99) proposed to be the P2Y15 receptor is not a genuine P2Y receptor. Trends Pharmacol Sci, 26 (1): 8-9. [PMID:15629198]
4. Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. (2003) P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest, 112 (3): 398-406. [PMID:12897207]
5. Bianco F, Fumagalli M, Pravettoni E, D'Ambrosi N, Volonté C, Matteoli M, Abbracchio MP, Verderio C. (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev, 48: 144-156. [PMID:15850653]
6. Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. (1997) Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem, 272 (19): 12583-90. [PMID:9139711]
7. Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. (1998) Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol, 124 (3): 428-30. [PMID:9647463]
8. Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. (2002) 2-Chloro N(6)-methyl-(N)-methanocarba-2'-deoxyadenosine-3',5'-bisphosphate is a selective high affinity P2Y(1) receptor antagonist. Br J Pharmacol, 135: 2004-2010. [PMID:11959804]
9. Boyer JL, Delaney SM, Villanueva D, Harden TK. (2000) A molecularly identified P2Y receptor simultaneously activates phospholipase C and inhibits adenylyl cyclase and is nonselectively activated by all nucleoside triphosphates. Mol Pharmacol, 57 (4): 805-10. [PMID:10727529]
10. Boyer JL, Waldo GL, Harden TK. (1997) Molecular cloning and expression of an avian G protein-coupled P2Y receptor. Mol Pharmacol, 52 (6): 928-34. [PMID:9415702]
11. Burnstock G. (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology, 36 (9): 1127-39. [PMID:9364468]
12. Burnstock GDobson. (1998) The G protein-coupled P2Y receptors. In Cardiovascular Biology of Purines Edited by Burnstock G (Kluwer Academic Publishers) 187-205. [ISBN:0792383346]
13. Cattaneo M, Gachet C. (1999) ADP receptors and clinical bleeding disorders. Arterioscler Thromb Vasc Biol, 19 (10): 2281-5. [PMID:10521355]
14. Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L et al.. (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem, 275 (15): 10767-71. [PMID:10753868]
15. Chang K, Hanaoka K, Kumada M, Takuwa Y. (1995) Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem, 270: 26152-26158. [PMID:7592819]
16. Charlton ME, Williams AS, Fogliano M, Sweetnam PM, Duman RS. (1997) The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res, 764 (1-2): 141-8. [PMID:9295203]
17. Communi D, Gonzalez NS, Detheux M, Brézillon S, Lannoy V, Parmentier M, Boeynaems JM. (2001) Identification of a novel human ADP receptor coupled to G(i). J Biol Chem, 276 (44): 41479-85. [PMID:11546776]
18. Communi D, Govaerts C, Parmentier M, Boeynaems JM. (1997) Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem, 272 (51): 31969-73. [PMID:9405388]
19. Communi D, Parmentier M, Boeynaems JM. (1996) Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun, 222 (2): 303-8. [PMID:8670200]
20. Communi D, Pirotton S, Parmentier M, Boeynaems JM. (1995) Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem, 270: 30849-30852. [PMID:8537336]
21. Costanzi S, Mamedova L, Gao ZG, Jacobson KA. (2004) Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem, 47: 5393-5404. [PMID:15481977]
22. Ennion SJ, Powell AD, Seward EP. (2004) Identification of the P2Y(12) receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol Pharmacol, 66 (3): 601-11. [PMID:15322252]
23. Erb L, Garrad R, Wang Y, Quinn T, Turner JT, Weisman GA. (1995) Site-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J Biol Chem, 270 (9): 4185-8. [PMID:7876172]
24. Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. (1999) Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med, 5 (10): 1199-202. [PMID:10502826]
25. Filippov AK, Brown DA, Barnard EA. (2000) The P2Y(1) receptor closes the N-type Ca(2+) channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. Br J Pharmacol, 129 (6): 1063-6. [PMID:10725253]
26. Fischer B, Boyer JL, Hoyle CH, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. (1993) Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5'-triphosphate. J Med Chem, 36 (24): 3937-46. [PMID:8254622]
27. Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma Jr FJ, Wiekowski MT et al.. (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest, 107 (12): 1591-8. [PMID:11413167]
28. Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. (1997) Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci, 18 (3): 79-82. [PMID:9133776]
29. Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K et al.. (2001) Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics, 78 (3): 124-8. [PMID:11735218]
30. Fumagalli M, Brambilla R, D'Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. (2003) Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia, 43: 218-203. [PMID:12898701]
31. Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP. (2004) Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y(13) receptor. Biochem Pharmacol, 68 (1): 113-24. [PMID:15183123]
32. Gachet C, Hechler B. (2005) The platelet P2 receptors in thrombosis. Semin Thromb Hemost, 31 (2): 162-7. [PMID:15852219]
33. Gordon JL. (1986) Extracellular ATP: effects, sources and fate. Biochem J, 233 (2): 309-19. [PMID:3006665]
34. Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. (1996) A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem, 271 (27): 16384-92. [PMID:8663163]
35. Hechler B, Eckly A, Ohlmann P, Cazenave J-P, Gachet C. (1998) The P2Y1 receptor, necessary but not sufficient to support full ADP-induced platelet aggregation, is not the target of the drug clopidogrel. Br J Haematol, 103: 858-866. [PMID:9858246]
36. Hechler B, Vigne P, Léon C, Breittmayer JP, Gachet C, Frelin C. (1998) ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol, 53 (4): 727-33. [PMID:9547364]
37. Herzog H, Darby K, Hort YJ, Shine J. (1996) Intron 17 of the human retinoblastoma susceptibility gene encodes an actively transcribed G protein-coupled receptor gene. Genome Res, 6 (9): 858-61. [PMID:8889552]
38. Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D et al.. (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature, 409 (6817): 202-7. [PMID:11196645]
39. Humphries RG. (2000) Pharmacology of AR-C69931MX and related compounds: from pharmacological tools to clinical trials. Haematologica, 85: 66-72.
40. Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA et al.. (1999) Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem, 42 (2): 213-20. [PMID:9925726]
41. Jacobson KA, Hoffmann C, Kim YC, Camaioni E, Nandanan E, Jang SY, Guo DP, Ji XD, von Kügelgen I, Moro S et al.. (1999) Molecular recognition in P2 receptors: ligand development aided by molecular modeling and mutagenesis. Prog Brain Res, 120: 119-32. [PMID:10550992]
42. Janssens R, Boeynaems JM, Godart M, Communi D. (1997) Cloning of a human heptahelical receptor closely related to the P2Y5 receptor. Biochem Biophys Res Commun, 236 (1): 106-12. [PMID:9223435]
43. Jiang Q, Guo D, Lee BX, Van Rhee AM, Kim YC, Nicholas RA, Schachter JB, Harden TK, Jacobson KA. (1997) A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol, 52 (3): 499-507. [PMID:9281613]
44. Jin J, Daniel JL, Kunapuli SP. (1998) Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem, 273 (4): 2030-4. [PMID:9442040]
45. Jordan BA, Devi LA. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature, 399 (6737): 697-700. [PMID:10385123]
46. Kennedy C, Herold CL, Qi A, Nicholas RA, Harden TK. (1999) Differing pharmacological properties of the human and rat P2Y4 receptors. Br J Pharmacol, 126: 21P.
47. Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. (2000) ATP, an agonist at the rat P2Y(4) receptor, is an antagonist at the human P2Y(4) receptor. Mol Pharmacol, 57 (5): 926-31. [PMID:10779375]
48. King BF, Townsend-Nicholson A, Burnstock G. (1998) Metabotropic receptors for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. Trends Pharmacol Sci, 19: 506-514. [PMID:9871413]
49. Kobayashi T, Soma T, Noguchi T, Nakagome K, Nakamoto H, Kita H, Nagata M. (2015) ATP drives eosinophil effector responses through P2 purinergic receptors. Allergol Int, 64 Suppl: S30-6. [PMID:26344078]
50. Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. (1997) Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci USA, 94 (3): 831-6. [PMID:9023342]
51. Kunapuli SP. (1998) Functional characterization of platelet ADP receptors. Platelets, 9 (6): 343-51. [PMID:16793717]
52. Li Q, Olesky M, Palmer RK, Harden TK, Nicholas RA. (1998) Evidence that the p2y3 receptor is the avian homologue of the mammalian P2Y6 receptor. Mol Pharmacol, 54 (3): 541-6. [PMID:9730913]
53. Li Q, Schachter JB, Harden TK, Nicholas RA. (1997) The 6H1 orphan receptor, claimed to be the p2y5 receptor, does not mediate nucleotide-promoted second messenger responses. Biochem Biophys Res Commun, 236 (2): 455-60. [PMID:9240460]
54. Lustig KD, Shiau AK, Brake AJ, Julius D. (1993) Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA, 90 (11): 5113-7. [PMID:7685114]
55. Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. (1999) Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest, 104 (12): 1731-7. [PMID:10606627]
56. Léon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C. (1997) The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett, 403 (1): 26-30. [PMID:9038354]
57. Mamedova LK, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA. (2004) Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol, 67 (9): 1763-70. [PMID:15081875]
58. Marchese A, George SR, Kolakowski Jr LF, Lynch KR, O'Dowd BF. (1999) Novel GPCRs and their endogenous ligands: expanding the boundaries of physiology and pharmacology. Trends Pharmacol Sci, 20 (9): 370-5. [PMID:10462760]
59. Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. (2003) Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol, 64 (1): 104-12. [PMID:12815166]
60. Meyer MP, Clarke JD, Patel K, Townsend-Nicholson A, Burnstock G. (1999) Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn, 214 (2): 152-8. [PMID:10030594]
61. Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR. (2001) Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta, 1521 (1-3): 107-19. [PMID:11690642]
62. Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC. (2003) GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res, 118 (1-2): 10-23. [PMID:14559350]
63. Nandanan E, Camaioni E, Jang SY, Kim YC, Cristalli G, Herdewijn P, Secrist 3rd JA, Tiwari KN, Mohanram A, Harden TK et al.. (1999) Structure-activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J Med Chem, 42 (9): 1625-38. [PMID:10229631]
64. Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK et al.. (2000) Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y(1) receptor ligands. J Med Chem, 43 (5): 829-42. [PMID:10715151]
65. Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. (1996) Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci, 19 (1): 13-8. [PMID:8787135]
66. Nguyen T, Erb L, Weisman GA, Marchese A, Heng HH, Garrad RC, George SR, Turner JT, O'Dowd BF. (1995) Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem, 270: 30845-30848. [PMID:8537335]
67. Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. (1996) Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol, 50 (2): 224-9. [PMID:8700127]
68. Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. (1998) Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol, 54 (6): 1118-23. [PMID:9855642]
69. Park MK, Garrad RC, Weisman GA, Turner JT. (1997) Changes in P2Y1 nucleotide receptor activity during the development of rat salivary glands. Am J Physiol, 272 (4 Pt 1): C1388-93. [PMID:9142866]
70. Pelleg A, Schulman ES, Barnes PJ. (2016) Extracellular Adenosine 5'-Triphosphate in Obstructive Airway Diseases. Chest, 150 (4): 908-915. [PMID:27568579]
71. Pintor J, Díaz-Rey MA, Miras-Portugal MT. (1993) Ap4A and ADP-beta-S binding to P2 purinoceptors present on rat brain synaptic terminals. Br J Pharmacol, 108 (4): 1094-9. [PMID:8485620]
72. Pintor J, Miras-Portugal MT. (1995) P2 purinergic receptors for diadenosine polyphosphates in the nervous system. Gen Pharmacol, 26 (2): 229-35. [PMID:7590071]
73. Pintor J, Miras-Portugal MT. (2000) Receptors for diadenosine polyphosphates P2D, P2YApnA, P4 and dinucleotide receptors: are there too many?. Trends Pharmacol Sci, 21 (4): 135. [PMID:10740288]
74. Ralevic V, Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol Rev, 50: 413-492. [PMID:9755289]
75. Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. (2003) Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia, 44: 242-250. [PMID:14603465]
76. Savi P, Labouret C, Delesque N, Guette F, Lupker J, Herbert JM. (2001) P2y(12), a new platelet ADP receptor, target of clopidogrel. Biochem Biophys Res Commun, 283 (2): 379-83. [PMID:11327712]
77. Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. (1996) Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br J Pharmacol, 118 (1): 167-73. [PMID:8733591]
78. Schwarz UR, Geiger J, Walter U, Eigenthaler M. (1999) Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost, 82 (3): 1145-52. [PMID:10494779]
79. Shaver SR, Rideout JL, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M et al.. (2005) Structure-activity relationships of dinucleotides: Potent and selective agonists of P2Y receptors. Purinergic Signal, 1 (2): 183-91. [PMID:18404503]
80. Simon J, Vigne P, Eklund KM, Michel AD, Carruthers AM, Humphrey PP, Frelin C, Barnard EA. (2001) Activity of adenosine diphosphates and triphosphates on a P2Y(T) -type receptor in brain capillary endothelial cells. Br J Pharmacol, 132 (1): 173-82. [PMID:11156575]
81. van Rhee AM, Fischer B, Van Galen PJ, Jacobson KA. (1995) Modelling the P2Y purinoceptor using rhodopsin as template. Drug Des Discov, 13: 133-154. [PMID:8872457]
82. Wang L, Jacobsen SE, Bengtsson A, Erlinge D. (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol, 5: 16. [PMID:15291969]
83. Webb TE, Henderson D, King BF, Wang S, Simon J, Bateson AN, Burnstock G, Barnard EA. (1996) A novel G protein-coupled P2 purinoceptor (P2Y3) activated preferentially by nucleoside diphosphates. Mol Pharmacol, 50 (2): 258-65. [PMID:8700132]
84. Webb TE, Henderson DJ, Roberts JA, Barnard EA. (1998) Molecular cloning and characterization of the rat P2Y4 receptor. J Neurochem, 71: 1348-1357. [PMID:9751165]
85. Webb TE, Kaplan MG, Barnard EA. (1996) Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun, 219 (1): 105-10. [PMID:8619790]
86. Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. (1993) Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett, 324: 219-225. [PMID:8508924]
87. Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D. (2004) ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol, 24 (10): 1810-5. [PMID:15308557]
88. Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J et al.. (2002) Pharmacology of INS37217 [P(1)-(uridine 5')-P(4)- (2'-deoxycytidine 5')tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther, 302 (3): 871-80. [PMID:12183642]
89. Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. (1997) A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature, 387 (6633): 620-4. [PMID:9177352]
90. Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, Yang S, Laz TM, Bayne M, Monsma Jr F. (2002) P2Y(13): identification and characterization of a novel Galphai-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther, 301 (2): 705-13. [PMID:11961076]






