GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
cyclin dependent kinase 4
Target not currently curated in GtoImmuPdb
Target id: 1976
Nomenclature: cyclin dependent kinase 4
Abbreviated Name: CDK4
Family: CDK4 subfamily
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 303 | 12q14.1 | CDK4 | cyclin dependent kinase 4 | |
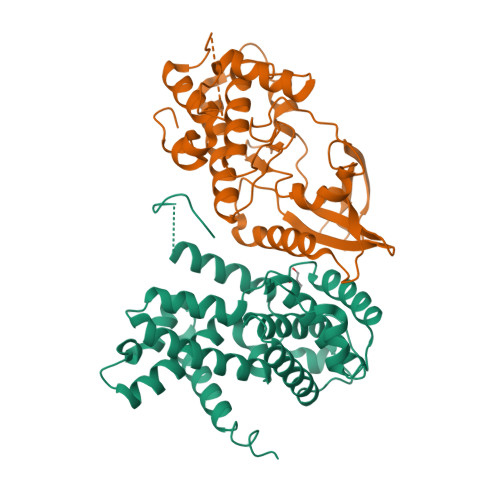
| Mouse | - | 303 | 10 D3 | Cdk4 | cyclin dependent kinase 4 | |
| Rat | - | 303 | 7q22 | Cdk4 | cyclin-dependent kinase 4 | |
Previous and Unofficial Names  |
|
cell division protein kinase 4 | CRK3 | p34 |
Database Links  |
|
| Alphafold | P11802 (Hs), P30285 (Mm), P35426 (Rn) |
| BRENDA | 2.7.11.22 |
| ChEMBL Target | CHEMBL331 (Hs), CHEMBL2134 (Mm) |
| Ensembl Gene | ENSG00000135446 (Hs), ENSMUSG00000006728 (Mm), ENSRNOG00000025602 (Rn) |
| Entrez Gene | 1019 (Hs), 12567 (Mm), 94201 (Rn) |
| Human Protein Atlas | ENSG00000135446 (Hs) |
| KEGG Enzyme | 2.7.11.22 |
| KEGG Gene | hsa:1019 (Hs), mmu:12567 (Mm), rno:94201 (Rn) |
| OMIM | 123829 (Hs) |
| Orphanet | ORPHA119289 (Hs) |
| Pharos | P11802 (Hs) |
| RefSeq Nucleotide | NM_000075 (Hs), NM_009870 (Mm), NM_053593 (Rn) |
| RefSeq Protein | NP_000066 (Hs), NP_034000 (Mm), NP_446045 (Rn) |
| UniProtKB | P11802 (Hs), P30285 (Mm), P35426 (Rn) |
| Wikipedia | CDK4 (Hs) |
Selected 3D Structures  |
|||||||||||
|
|
||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inhibitor Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fascaplysin did not inhibit CDK4/cyclin D2 and CDK4/cyclin D3 at concentrations >100μM, but did inhibit CDK6/cyclin D2 but with a low affinity of 35000nM [32]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 11,39 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: CDK4-cyclinD1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: CDK4-cyclinD3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: ...1 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: nd/CDK4/cyclin D1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: nd/CDK4/cyclin D3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
References
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
2. Behenna DC, Chen P, Freeman-Cook KD, Hoffman RL, Jalaie M, Nagata A, Nair SK, Ninkovic S, Ornelas MA, Palmer CL et al.. (2018) Pyridopyrimdinone cdk2/4/6 inhibitors. Patent number: WO2018033815A1. Assignee: Pfizer Inc.. Priority date: 15/08/2016. Publication date: 22/02/2018.
3. Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC. (2016) Preclinical Characterization of G1T28: A Novel CDK4/6 Inhibitor for Reduction of Chemotherapy-Induced Myelosuppression. Mol Cancer Ther, 15 (5): 783-93. [PMID:26826116]
4. Brain CT, Sung MJ, Lago B. (2015) Pyrrolopyrimidine compounds and their uses. Patent number: US8962630. Assignee: Novartis Ag. Priority date: 22/08/2008. Publication date: 24/02/2015.
5. Brasca MG, Amboldi N, Ballinari D, Cameron A, Casale E, Cervi G, Colombo M, Colotta F, Croci V, D'Alessio R et al.. (2009) Identification of N,1,4,4-tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide (PHA-848125), a potent, orally available cyclin dependent kinase inhibitor. J Med Chem, 52 (16): 5152-63. [PMID:19603809]
6. Buesking AW, Combs AP, Zhou J, Holmes R, Pawley S, Wo X. (2022) Cdk inhibitors and their use as pharmaceuticals. Patent number: WO2022061273A1. Assignee: Prelude Therapeutics Inc. Priority date: 21/09/2021. Publication date: 24/03/2022.
7. Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. (1996) Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res, 56 (13): 2973-8. [PMID:8674031]
8. Changhee M, Oh B-g, Kim Y-e, Park C-m. (2016) Heterocyclic compound and pharmaceutical composition comprising same. Patent number: WO2016126085A2. Assignee: Beyond Bio. Priority date: 04/02/2015. Publication date: 03/11/2016.
9. Chen P, Cho-Schultz S, Deal JG, Gallego GM, Jalaie M, Kania RS, Nair SK, Ninlovic S, Orr STM, Palmer CL. (2020) Cyclin dependent kinase inhibitors. Patent number: US20200354350A1. Assignee: Pfizer Inc. Priority date: 29/07/2020. Publication date: 12/11/2020.
10. Cirstea D, Hideshima T, Santo L, Eda H, Mishima Y, Nemani N, Hu Y, Mimura N, Cottini F, Gorgun G et al.. (2013) Small-molecule multi-targeted kinase inhibitor RGB-286638 triggers P53-dependent and -independent anti-multiple myeloma activity through inhibition of transcriptional CDKs. Leukemia, 27 (12): 2366-75. [PMID:23807770]
11. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
12. Day PJ, Cleasby A, Tickle IJ, O'Reilly M, Coyle JE, Holding FP, McMenamin RL, Yon J, Chopra R, Lengauer C et al.. (2009) Crystal structure of human CDK4 in complex with a D-type cyclin. Proc Natl Acad Sci USA, 106 (11): 4166-70. [PMID:19237565]
13. DePinto W, Chu XJ, Yin X, Smith M, Packman K, Goelzer P, Lovey A, Chen Y, Qian H, Hamid R et al.. (2006) In vitro and in vivo activity of R547: a potent and selective cyclin-dependent kinase inhibitor currently in phase I clinical trials. Mol Cancer Ther, 5 (11): 2644-58. [PMID:17121911]
14. Ding Q, Gillespie P, Kim K, McComas WW, Perrotta A. (2006) Diaminothiazoles having antiproliferative activity. Patent number: US7094896. Assignee: Hoffmann-La Roche Inc.. Priority date: 22/12/2001. Publication date: 22/08/2006.
15. Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK et al.. (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther, 3 (11): 1427-38. [PMID:15542782]
16. Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack RS et al.. (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs, 32 (5): 825-37. [PMID:24919854]
17. Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L et al.. (1998) Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science, 281 (5376): 533-8. [PMID:9677190]
18. Hole AJ, Baumli S, Shao H, Shi S, Huang S, Pepper C, Fischer PM, Wang S, Endicott JA, Noble ME. (2013) Comparative structural and functional studies of 4-(thiazol-5-yl)-2-(phenylamino)pyrimidine-5-carbonitrile CDK9 inhibitors suggest the basis for isotype selectivity. J Med Chem, 56 (3): 660-70. [PMID:23252711]
19. Jiang B, Wang ES, Donovan KA, Liang Y, Fischer ES, Zhang T, Gray NS. (2019) Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angew Chem Int Ed Engl, 58 (19): 6321-6326. [PMID:30802347]
20. Jones CD, Andrews DM, Barker AJ, Blades K, Byth KF, Finlay MR, Geh C, Green CP, Johannsen M, Walker M et al.. (2008) Imidazole pyrimidine amides as potent, orally bioavailable cyclin-dependent kinase inhibitors. Bioorg Med Chem Lett, 18 (24): 6486-9. [PMID:18986805]
21. Jorda R, Havlíček L, Šturc A, Tušková D, Daumová L, Alam M, Škerlová J, Nekardová M, Peřina M, Pospíšil T et al.. (2019) 3,5,7-Substituted Pyrazolo[4,3- d]pyrimidine Inhibitors of Cyclin-Dependent Kinases and Their Evaluation in Lymphoma Models. J Med Chem, 62 (9): 4606-4623. DOI: 10.1021/acs.jmedchem.9b00189 [PMID:30943029]
22. Jorda R, Hendrychová D, Voller J, Řezníčková E, Gucký T, Kryštof V. (2018) How Selective Are Pharmacological Inhibitors of Cell-Cycle-Regulating Cyclin-Dependent Kinases?. J Med Chem, 61 (20): 9105-9120. [PMID:30234987]
23. Joshi KS, Rathos MJ, Joshi RD, Sivakumar M, Mascarenhas M, Kamble S, Lal B, Sharma S. (2007) In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Mol Cancer Ther, 6 (3): 918-25. [PMID:17363486]
24. Kubo A, Nakagawa K, Varma RK, Conrad NK, Cheng JQ, Lee WC, Testa JR, Johnson BE, Kaye FJ, Kelley MJ. (1999) The p16 status of tumor cell lines identifies small molecule inhibitors specific for cyclin-dependent kinase 4. Clin Cancer Res, 5 (12): 4279-86. [PMID:10632371]
25. Lane ME, Yu B, Rice A, Lipson KE, Liang C, Sun L, Tang C, McMahon G, Pestell RG, Wadler S. (2001) A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res, 61 (16): 6170-7. [PMID:11507069]
26. Liu S, Zhou Y, Wu X, Bao R. (2018) Benzimidazole compound kinase inhibitor, preparation method therefor and application thereof. Patent number: WO2018045956A1. Assignee: Jiangsu Haosen Pharmaceutical Group, Shanghai Hansen Biomedical Technology Co.. Priority date: 07/09/2016. Publication date: 15/03/2018.
27. Paiva C, Godbersen JC, Soderquist RS, Rowland T, Kilmarx S, Spurgeon SE, Brown JR, Srinivasa SP, Danilov AV. (2015) Cyclin-Dependent Kinase Inhibitor P1446A Induces Apoptosis in a JNK/p38 MAPK-Dependent Manner in Chronic Lymphocytic Leukemia B-Cells. PLoS ONE, 10 (11): e0143685. [PMID:26606677]
28. Pennati M, Campbell AJ, Curto M, Binda M, Cheng Y, Wang LZ, Curtin N, Golding BT, Griffin RJ, Hardcastle IR et al.. (2005) Potentiation of paclitaxel-induced apoptosis by the novel cyclin-dependent kinase inhibitor NU6140: a possible role for survivin down-regulation. Mol Cancer Ther, 4 (9): 1328-37. [PMID:16170024]
29. Pham SM, Chakravarty S, Kanlanala J, Pujala B, Shete A, Bhatt B, Agarwal AK, Soni S, Chen J. (2019) Heterocyclic compounds as kinase inhibitors. Patent number: WO2019161224A1. Assignee: GiraFpharma LLC. Priority date: 15/02/2018. Publication date: 22/08/2019.
30. Reddy MV, Akula B, Cosenza SC, Athuluridivakar S, Mallireddigari MR, Pallela VR, Billa VK, Subbaiah DR, Bharathi EV, Vasquez-Del Carpio R et al.. (2014) Discovery of 8-cyclopentyl-2-[4-(4-methyl-piperazin-1-yl)-phenylamino]-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidine-6-carbonitrile (7x) as a potent inhibitor of cyclin-dependent kinase 4 (CDK4) and AMPK-related kinase 5 (ARK5). J Med Chem, 57 (3): 578-99. [PMID:24417566]
31. Ryu CK, Kang HY, Lee SK, Nam KA, Hong CY, Ko WG, Lee BH. (2000) 5-Arylamino-2-methyl-4,7-dioxobenzothiazoles as inhibitors of cyclin-dependent kinase 4 and cytotoxic agents. Bioorg Med Chem Lett, 10 (5): 461-4. [PMID:10743948]
32. Soni R, Muller L, Furet P, Schoepfer J, Stephan C, Zumstein-Mecker S, Fretz H, Chaudhuri B. (2000) Inhibition of cyclin-dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem Biophys Res Commun, 275 (3): 877-84. [PMID:10973815]
33. Squires MS, Feltell RE, Wallis NG, Lewis EJ, Smith DM, Cross DM, Lyons JF, Thompson NT. (2009) Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent kinases, in human tumor cell lines. Mol Cancer Ther, 8 (2): 324-32. [PMID:19174555]
34. Strum JC, Bisi JE, Roberts PJ, Tavares FX. (2016) Transient protection of normal cells during chemotherapy. Patent number: US9464092B2. Assignee: G1 Therapeutics Inc. Priority date: 15/03/2013. Publication date: 11/10/2016.
35. Su S, Yang Z, Gao H, Yang H, Zhu S, An Z, Wang J, Li Q, Chandarlapaty S, Deng H et al.. (2019) Potent and Preferential Degradation of CDK6 via Proteolysis Targeting Chimera Degraders. J Med Chem, 62 (16): 7575-7582. [PMID:31330105]
36. Sun P, Lan J, Peng J, Chen Y, Wang B, Dong Q. (2014) Thiophene miazines derivate, preparation method therefor, and medical application thereof. Patent number: WO2014183520A1. Assignee: Shanghai Hengrui Pharmaceutical Co. Priority date: 17/05/2013. Publication date: 20/11/2014.
37. Tadesse S, Yu M, Mekonnen LB, Lam F, Islam S, Tomusange K, Rahaman MH, Noll B, Basnet SK, Teo T et al.. (2017) Highly Potent, Selective, and Orally Bioavailable 4-Thiazol-N-(pyridin-2-yl)pyrimidin-2-amine Cyclin-Dependent Kinases 4 and 6 Inhibitors as Anticancer Drug Candidates: Design, Synthesis, and Evaluation. J Med Chem, 60 (5): 1892-1915. [PMID:28156111]
38. Wang Y, Zhang C, Wang J, Ding L. (2021) Crystal form of compound for inhibiting the activity of cdk4/6 and use thereof. Patent number: US20210261546A1. Assignee: Betta Pharmaceuticals Co Ltd. Priority date: 21/06/2019. Publication date: 26/08/2021.
39. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
40. Yin L, Li H, Liu W, Yao Z, Cheng Z, Zhang H, Zou H. (2018) A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in gliobastoma therapy. Eur J Med Chem, 144: 1-28. [PMID:29247857]
41. Zhu G, Conner SE, Zhou X, Shih C, Li T, Anderson BD, Brooks HB, Campbell RM, Considine E, Dempsey JA et al.. (2003) Synthesis, structure-activity relationship, and biological studies of indolocarbazoles as potent cyclin D1-CDK4 inhibitors. J Med Chem, 46 (11): 2027-30. [PMID:12747775]
How to cite this page
CDK4 subfamily: cyclin dependent kinase 4. Last modified on 06/11/2025. Accessed on 06/03/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1976.