|
Compound class:
Synthetic organic
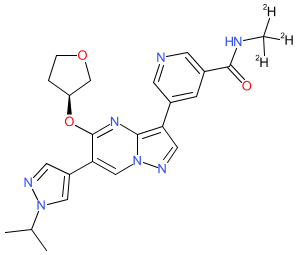
Comment: This compound is a receptor tyrosine kinase inhibitor with selectivity for fibroblast growth factor receptors 2 and 3 (FGFR2/3) [2]. It is active at both the wild type kinases and at those with activiating mutations in the gatekeeper region (e.g. FGFR2V564I/L/M/F, FGFR3V555I/L/M/F) that are associated with malignancies such as cholangiocarcinoma and bladder cancer. Erdafitinib, pemigatinib and infigratinib are drugs that are already used clinically to target abberant FGFR signalling in specific solid tumour types. These are all pan-FGFR inhibitors and can cause hyperphosphatemia, most likely via activation of FGFR1 [1,3], so sparing this receptor is hypothesised to improve the toxicity profile of FGFR inhibiting drugs.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel