GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: KAE609 | NITD609
Compound class:
Synthetic organic
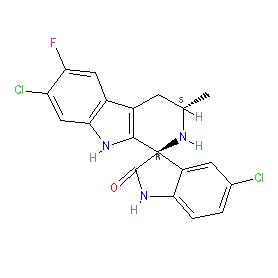
Comment: Cipargamin is an antimalarial drug candidate, under clinical development. It is the optimised lead for the spiroindolones, a novel class of compounds identified from a phenotypic screen as having antimalarial activity [3,9] and awarded MMV Project of the Year (2009).
The active enantiomer is shown here, with the 1R,3S configuration essential for antimalarial activity [3]. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Goldgof GM, Durrant JD, Ottilie S, Vigil E, Allen KE, Gunawan F, Kostylev M, Henderson KA, Yang J, Schenken J et al.. (2016)
Comparative chemical genomics reveal that the spiroindolone antimalarial KAE609 (Cipargamin) is a P-type ATPase inhibitor. Sci Rep, 6: 27806. [PMID:27291296] |
|
2. Hien TT, White NJ, Thuy-Nhien NT, Hoa NT, Thuan PD, Tarning J, Nosten F, Magnusson B, Jain JP, Hamed K. (2017)
Estimation of the In Vivo MIC of Cipargamin in Uncomplicated Plasmodium falciparum Malaria. Antimicrob Agents Chemother, 61 (2). [PMID:27872070] |
|
3. Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J et al.. (2010)
Spiroindolones, a potent compound class for the treatment of malaria. Science, 329 (5996): 1175-80. [PMID:20813948] |
|
4. Schmitt EK, Ndayisaba G, Yeka A, Asante KP, Grobusch MP, Karita E, Mugerwa H, Asiimwe S, Oduro A, Fofana B et al.. (2022)
Efficacy of Cipargamin (KAE609) in a Randomized, Phase II Dose-Escalation Study in Adults in Sub-Saharan Africa With Uncomplicated Plasmodium falciparum Malaria. Clin Infect Dis, 74 (10): 1831-1839. [PMID:34410358] |
|
5. Spillman NJ, Allen RJ, McNamara CW, Yeung BK, Winzeler EA, Diagana TT, Kirk K. (2013)
Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe, 13 (2): 227-37. [PMID:23414762] |
|
6. Stein DS, Jain JP, Kangas M, Lefèvre G, Machineni S, Griffin P, Lickliter J. (2015)
Open-label, single-dose, parallel-group study in healthy volunteers to determine the drug-drug interaction potential between KAE609 (cipargamin) and piperaquine. Antimicrob Agents Chemother, 59 (6): 3493-500. [PMID:25845867] |
|
7. van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. (2012)
The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother, 56 (7): 3544-8. [PMID:22508309] |
|
8. White NJ, Pukrittayakamee S, Phyo AP, Rueangweerayut R, Nosten F, Jittamala P, Jeeyapant A, Jain JP, Lefèvre G, Li R et al.. (2014)
Spiroindolone KAE609 for falciparum and vivax malaria. N Engl J Med, 371 (5): 403-10. [PMID:25075833] |
|
9. Yeung BK, Zou B, Rottmann M, Lakshminarayana SB, Ang SH, Leong SY, Tan J, Wong J, Keller-Maerki S, Fischli C et al.. (2010)
Spirotetrahydro beta-carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J Med Chem, 53 (14): 5155-64. [PMID:20568778] |