GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: KAE609 | NITD609
Compound class:
Synthetic organic
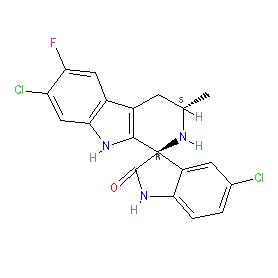
Comment: Cipargamin is an antimalarial drug candidate, under clinical development. It is the optimised lead for the spiroindolones, a novel class of compounds identified from a phenotypic screen as having antimalarial activity [3,9] and awarded MMV Project of the Year (2009).
The active enantiomer is shown here, with the 1R,3S configuration essential for antimalarial activity [3]. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| The interaction table below provides data from whole cell assays and gives the antiparasite activity of cipargamin against a number of Plasmodium lifecycle stages. The results include an evaluation of asexual blood stage activity using a panel of culture-adapted P. falciparum strains, with cipargamin demonstrating no evidence of reduced potency against drug resistant strains. In addition, ex vivo testing of field-isolates from regions with known chloroquine resistance has shown potent asexual blood stage activity for both P. falciparum and P. vivax (IC50 <10nM) [3]. |
| Whole organism assay data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click on species/strain names for details | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||