|
Synonyms: ASP-2215 | ASP2215 | Xospata®
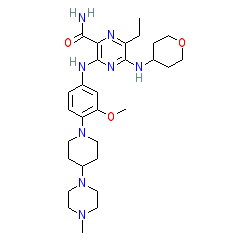
gilteritinib is an approved drug (FDA (2018), EMA (2019))
Compound class:
Synthetic organic
Comment: Gilteritinib is an orally bioavailable inhibitor of the receptor tyrosine kinases (RTKs) FMS-related tyrosine kinase 3 (FLT3), AXL and anaplastic lymphoma kinase (ALK) [4], with clinical antineoplastic activity. Gilteritinib inhibits the activity of FLT3-activating mutations which are one of the most common genetic alterations in acute myeloid leukemia (AML).
SARS-CoV-2: AXL is a kinase upstream of p38 MAP kinases. p38 activity has been reported to be upregulated following SARS-CoV-2 infection of host cells in vitro, and gilteritinib produces an antiviral effect (IC50= 807 nM) [1]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Marrero MC, Polacco BJ, Melnyk JE, Ulferts S, Kaake RM. (2020)
The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell, Article Online Now. DOI: 10.1016/j.cell.2020.06.034 |
|
2. EMA.
Gilteritinib orphan designation. Accessed on 15/04/2019. Modified on 15/04/2019. EMA, https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3171961 |
|
3. EMA.
Xospata: CHMP summary of opinion. Accessed on 24/09/2019. Modified on 24/09/2019. European Medicines Agency, https://www.ema.europa.eu/en/medicines/human/summaries-opinion/xospata |
|
4. Lee LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, Small D, Levis M. (2017)
Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood, 129 (2): 257-260. [PMID:27908881] |
|
5. Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, Claxton D, Erba HP, Gill S, Goldberg S et al.. (2017)
Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol, 18 (8): 1061-1075. [PMID:28645776] |