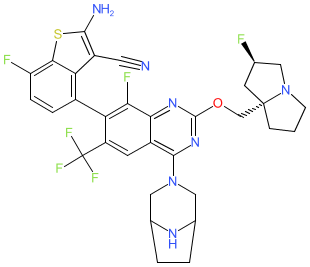
|
Synonyms: compound 14 [PMID: 37849557] | ERAS5024
Compound class:
Synthetic organic
Comment: ERAS-5024 was designed to target the KRAS G12D mutation in pancreatic ductal adenocarcinoma (PDAC) [2]. In vivo activity in PDAC xenograft mouse models suggested clinical potential. However, development was discontinued due to toxicity (histamine release and a pseudo-allergic reaction) caused by off-target agonist activity at the orphan GPCR, MAS related GPR family member X2 (MRGPRX2) [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Brooun A, Zhang J, Li C, Lam R, Cheng H, Shoemaker R, Daly J, Olaharski A. (2023)
The pharmacologic and toxicologic characterization of the potent and selective KRAS G12D inhibitors ERAS-4693 and ERAS-5024. Toxicol Appl Pharmacol, 474: 116601. [PMID:37321326] |
|
2. Cheng H, Li P, Chen P, Irimia A, Bae JH, Brooun A, Fagan P, Lam R, Lin B, Zhang J et al.. (2023)
Structure-Based Design and Synthesis of Potent and Selective KRAS G12D Inhibitors. ACS Med Chem Lett, 14 (10): 1351-1357. [PMID:37849557] |