GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
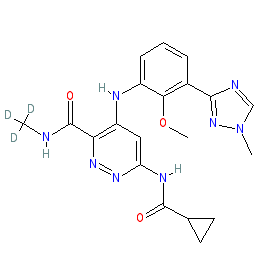
Synonyms: BMS-986165 | BMS986165 | compound 11 [PMID: 31318208} | Sotyktu® | Tyk2-IN-4
deucravacitinib is an approved drug (FDA (2022), EMA (2023))
Compound class:
Synthetic organic
Comment: Deucravacitinib (BMS-986165) is a selective, orally active, allosteric inhibitor of the Janus kinase family enzyme, tyrosine kinase 2 (TYK2) [2,5]. The deuteromethyl amide group confers selectivity by virtue of binding to a pocket in the TYK2 JH2 pseudokinase domain.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| TYK2 associates with the IL-23 cytokine receptor to induce STAT-mediated regulation of genes that are involved in expansion and maintenance of TH17 and TC17 cells, and this pathway is activated in and drives pathology in psoriatic disease. TYK2-selective inhibitors are proposed to facilitate targeted disruption this cytokine-kinase-T cell axis in psoriasis [4]. Deucravacitinib (BMS-986165) achieves its selectivity by targeting the JH2 pseudokinase domain of TYK2 rather than its orthosteric ATP binding domain [5]. It was the first pseudokinase-directed therapeutic to reach clinical evaluation as an oral treatment for autoimmune diseases. It has exhibited efficacy in several murine models of autoimmune disease and is reported to block signalling and functional responses in human cells that are known to drive autoimmune pathology (e.g.Th17, Th1, B cells, and myeloid cells) [1]. It was (FDA) approved to treat plaque psoriasis in 2022. |