GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
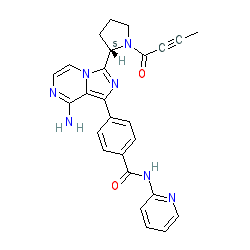
Synonyms: ACP-196 | Calquence® | Example 6 [US20140155385 A1] [2]
acalabrutinib is an approved drug (FDA (2017), EMA (2020))
Compound class:
Synthetic organic
Comment: Acalabrutinib is an orally available second-generation, selective and irreversible inhibitor of Bruton tyrosine kinase (BTK) [6], being investigated for its potential antineoplastic activity (in multiple haematologic malignancies and solid tumours), as well as a potential therapy for rheumatoid arthritis. Acalabrutinib covalently bonds to Cysteine-481 in BTK.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Having already received FDA Orphan Drug Designation and Breakthrough Therapy Designation for mantle cell lymphoma (MCL: a rare and fast-growing type of non-Hodgkin lymphoma), in August 2017 the FDA granted priority review for acalabrutinib's New Drug Application (NDA), based on results from a Phase 2 study in relapsed/refractory MCL (NCT02213926). This resulted in full FDA approval in October 2017 (link to FDA announcement). This approval is for the treatment of MCL patients who have received at least one prior therapy. For a list of all registered acalabrutinib trials, link here to ClinicalTrials.gov. In November 2019, FDA approval was expanded to include treatment of CLL or SLL, following evaluation in trials including NCT02475681 and NCT02970318; clinial trial results in patients with CLL are reported in [4], [3] and [1]. Trials to assess acalabrutinib's efficacy in a variety of solid tumours (such as bladder, prostate and non-small cell lung cancers) are ongoing. In the European Union, the EMA has granted acalabrutinib orphan designation for three rare diseases (as of 2016): CLL/SLL, lymphoplasmacytic lymphoma and MCL. SARS-CoV-2 and COVID-19: In response to the SARS-CoV-2 pandemic acalabrutinib was evaluated in COVID-19 patients, as part of the UK's Accelerating COVID-19 Research and Development (ACCORD) initiative (June 2020). ACCORD is designed to fast-track potential treatments for COVID-19 through early-stage clinical trials [5]. In this setting researchers would aimed to determine if the anti-inflammatory action of BTK-inhibition has efficacy to reduce mortality in patients with severe COVID-19. In November 2020, AstraZeneca announced that acalabrutinib missed its primary endpoint in Phase 2, and failed to "increase the proportion of patients who remained alive and free of respiratory failure". |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02213926 | An Open-label, Phase 2 Study of ACP-196 (Acalabrutinib) in Subjects With Mantle Cell Lymphoma | Phase 2 Interventional | Acerta Pharma BV | ||
| NCT02475681 | Elevate CLL TN: Study of Obinutuzumab + Chlorambucil, Acalabrutinib (ACP-196) + Obinutuzumab, and Acalabrutinib in Subjects With Previously Untreated CLL | Phase 3 Interventional | Acerta Pharma BV | ||
| NCT02970318 | A Study of Acalabrutinib vs Investigator's Choice of Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in R/R CLL | Phase 3 Interventional | Acerta Pharma BV | ||
| NCT04346199 | Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19. | Phase 2 Interventional | AstraZeneca | ||
| NCT04380688 | Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19. | Phase 2 Interventional | AstraZeneca | ||