GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
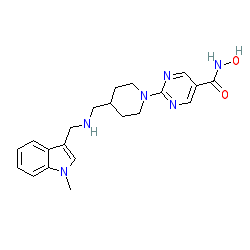
Synonyms: JNJ-26481585
Compound class:
Synthetic organic
Comment: Quisinostat is an HDAC inhibitor. The compound has highest potency for HDAC1 and modest potency with HDACs 2, 4, 10, and 11. Quisinostat exhibits >30-fold selectivity against HDACs 3, 5, 8, and 9, with lowest potency for HDACs 6 and 7 [1].
The compound also has antimalarial activity [2]. The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| This compound is being assessed in clinical trials for efficacy against solid and hematologic malignancies and has progressed to Phase 2 evaluation. Click here to link to ClinicalTrials.gov's full list of quisinostat studies. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Preclinical studies in human multiple myeloma cells (cell lines and from patient samples) show that quisinostat induces myeloma cell death at low nanomolar concentrations, via histone acetylation and by driving cells towards apoptisis [3-4]. |