GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: BD-40A | CGP-25827A | Foradil® | Oxeze/Oxis® | YM-08316
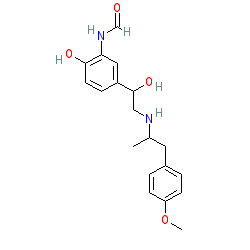
formoterol is an approved drug (FDA (2001))
Compound class:
Synthetic organic
Comment: Formoterol is a long-acting inhalation bronchodilator. The approved drug is a racemic mixture of enantiomers; PubChem lists 6 CIDs as isotopes of this molecule. We show the non-chiral molecule here to represent the mixture. The marketed formulation may contain formoterol fumarate (PubChem CID 9912089). Arformoterol is the (R,R)-enantiomer of formoterol.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: formoterol |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Formoterol is used to manage the symptoms of asthma and/or chronic obstructive pulmonary disease (COPD). In November 2014, the EU EMA granted marketing authorisation of the fixed-dose combination medication Duaklir Genuair TM (aclidinium bromide/formoterol fumarate) as a maintenance bronchodilator for patients with COPD. In April 2016, the US FDA approved the fixed-dose inhalation aerosol formulation, glycopyrrolate plus formoterol (Bevespi Aerosphere®) for long-term, maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Formoterol is a β2-adrenoceptor agonist. The drug causes vasodilation of bronchial smooth muscle to improve breathing. |