|
Synonyms: IFB-088 | IFB088 | Sephin-1 | Sephin1
Compound class:
Synthetic organic
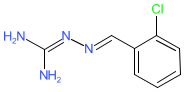
Comment: Icerguastat (IFB-088, Sephin1) selectively inhibits the regulatory subunit PPP1R15A within the PPP1R15A/PP1c phosphatase complex [2]. Inhibiting PPP1R15A results in reduced dephosphorylation of the translation initiation factor eIF2α, which rescues cells from proteostasis collapse. Structurally icerguastat is a guanabenz derivative but it has no α2-adrenerceptor agonist activity. Icerguastat is designed to maintain an active integrated stress response as a cytoprotective mechanism to combat the toxic effects of cellular stress [1].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05508074 | Treatment Combining Riluzole and IFB-088 in Bulbar Amyotrophic Lateral Sclerosis (TRIALS Protocol) | Phase 2 Interventional | InFlectis BioScience | ||
| NCT03610334 | A Combined SAD and MAD Study to Investigate the Safety, Tolerability and Pharmacokinetic Profile of IFB-088 | Phase 1 Interventional | InFlectis BioScience | ||