|
Synonyms: SB 939 | SB-939
Compound class:
Synthetic organic
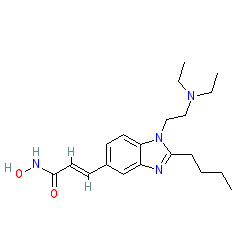
Comment: Pracinostat is an orally available, potent pan-HDAC inhibitor [3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Pracinostat does not inhibit HDAC6 (IC50 1µM) or the class III isoenzyme SIRT1 (IC50 >100µM) [3]. We have included the four highest potency interactions in the table below, and have tagged HDAC10 as the primary target (as it is inhibited most by pracinostat) for data metrics purposes only. The IC50 values for the remaining HDACs range from 56-140nM and can be obtained from Novotny-Diermayr et al (2010) [3]. |
| Selectivity at enzymes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||