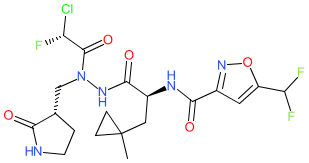
|
Compound class:
Synthetic organic
Comment: This compound is a coronavirus 3CL proteinase (3CLpro, Mpro) inhibitor from Hoffman La Roche's patent WO2022043374 [1]. It exhibits potent inhibitory activity at 3CLpro enzymes from a range of human coronaviruses (hCoV-229E, MERS-CoV, hCoV-OC43, SARS-CoV and SARS-CoV-2) in vitro. We present this as one of the most potent broad-range inhibitors from this patent. Extensive experimental work (in suitable cell lines, virus infection models etc.) would be required to determine which example (if any) is suitable for preclinical evaluation.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Inhibitory constants using recombinant 3CLpro enzymes from human coronaviruses in addition to the two SARS viruses were determined in in vitro assays [1]: hCoV-229E IC50 28 nM MERS-CoV IC50 94 nM hCoV-OC43 IC50 17 nM |
| Selectivity at other protein targets | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||