|
Synonyms: ATI-450 | ATI450 | CDD-450 | CDD450
Compound class:
Synthetic organic
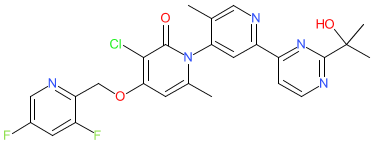
Comment: We obtained the chemical structure for zunsemetinib from WHO Proposed list 125 of July 2021. This mapped to PubChem CID 86291496. The compound is claimed in Confluence Life Sciences' patent US9115089B2 as kinase inhibitor with potential to treat p38 kinase-mediated diseases [2]. Confluence Life Sciences is a subsidiary of Aclaris Therapeutics. We speculate that zunsemetinib is the MAPK-activated protein kinase 2 (MK2) inhibitor ATI-450 (formerly CDD-450 [3]), that is included on Aclaris' pipeline page. Phase 1 safety and tolerability of ATI-450 were reported in June 2021 [1]. This article described ATI-450 as an inhibitor of p38α/MK2 pathway-mediated inflammatory signalling, but discloses only a partial chemical structure in a supplementary figure. The same incomplete chemical structure appears in [3]. ATI-450 binds with high affinity to the interface of the p38MAPK-MK2 complex and selectively inhibits p38MAPK-catalysed phosphorylation of MK2 which stabilises the inactive conformation of MK2, and subsequently reduces inflammatory cytokine levels [3].
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Zunsemetinib (CDD-450) binds with higher affinity to the p38α/MK2 complex than it does to either kinase alone. It is highly selective across the human kinome, being >350-fold more potent at p38α or p38β compared to a panel of >190 other kinases [3]. CDD-450 inhibits LPS-induced production of TNF-α and IL-1β (IC50 1-10 nM) and IL-6 (IC50 ~100 nM) in in vitro assays and animal models. |