GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Agonists
- Antagonists
- Antibodies
- Immunopharmacology Comments
- Immuno Disease Associations
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Functions
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Clinically-Relevant Mutations and Pathophysiology
- Biologically Significant Variants
- General Comments
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 352 | 2q22.1 | CXCR4 | C-X-C motif chemokine receptor 4 | 16,43 |
| Mouse | 7 | 359 | 1 56.43 cM | Cxcr4 | C-X-C motif chemokine receptor 4 | 23,49 |
| Rat | 7 | 349 | 13q13 | Cxcr4 | C-X-C motif chemokine receptor 4 | 33,68 |
Previous and Unofficial Names  |
|
| fusin [8,16,23] | LCR1 [68] | LESTR [43,49] | HM89 | HUMSTSR | CD184 | NPY3R | NPYY3R | CXCR-4 | CXC-R4 | SDF-1 receptor | stromal cell-derived factor 1 receptor | Cmkar4 | HSY3RR | chemokine (C-X-C motif) receptor 4 | |
Database Links  |
|
| Specialist databases | |
| GPCRdb | cxcr4_human (Hs), cxcr4_mouse (Mm), cxcr4_rat (Rn) |
| Other databases | |
| Alphafold | P61073 (Hs), P70658 (Mm), O08565 (Rn) |
| ChEMBL Target | CHEMBL2107 (Hs), CHEMBL1250365 (Mm), CHEMBL5556 (Rn) |
| DrugBank Target | P61073 (Hs) |
| Ensembl Gene | ENSG00000121966 (Hs), ENSMUSG00000045382 (Mm), ENSRNOG00000003866 (Rn) |
| Entrez Gene | 7852 (Hs), 12767 (Mm), 60628 (Rn) |
| Human Protein Atlas | ENSG00000121966 (Hs) |
| KEGG Gene | hsa:7852 (Hs), mmu:12767 (Mm), rno:60628 (Rn) |
| OMIM | 162643 (Hs) |
| Orphanet | ORPHA120914 (Hs) |
| Pharos | P61073 (Hs) |
| RefSeq Nucleotide | NM_003467 (Hs), NM_009911 (Mm), NM_022205 (Rn) |
| RefSeq Protein | NP_003458 (Hs), NP_034041 (Mm), NP_071541 (Rn) |
| SynPHARM |
6612 (in complex with isothiourea-1t) 6614 (in complex with isothiourea-1t) |
| UniProtKB | P61073 (Hs), P70658 (Mm), O08565 (Rn) |
| Wikipedia | CXCR4 (Hs) |
Selected 3D Structures  |
|||||||||||||
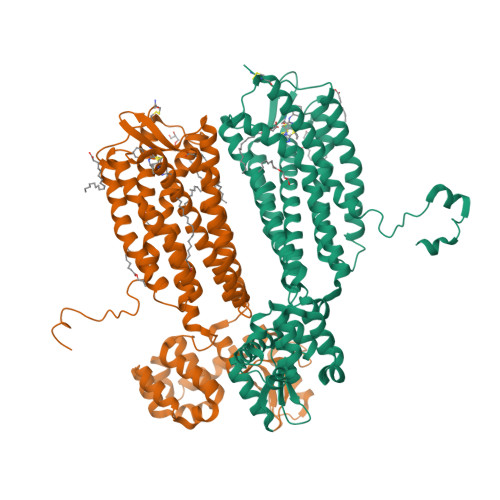
|
|
||||||||||||
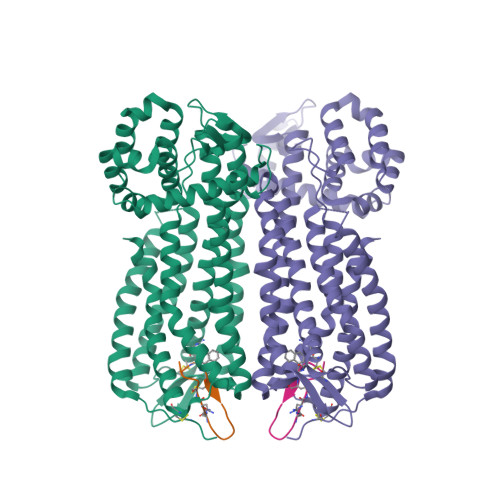
|
|
||||||||||||
Natural/Endogenous Ligands  |
| CXCL12γ {Sp: Human} |
| CXCL12δ {Sp: Human} |
| CXCL12ε {Sp: Human} |
| CXCL12φ {Sp: Human} |
| CXCL12β {Sp: Human} |
| CXCL12α {Sp: Human} |
| CXCL12 {Sp: Mouse} |
| Comments: SDF1α and SDF1β are the active isomers of CXCL12 |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDF-1α and SDF-1β are both referred to as CXCL12 and they have equivalent binding affinity and potency [26]. The CXCL12 isoforms (δ, γ, ε and φ) identified by Yu et al. (2006) were shown to stimulate cell migration in a CXCR4-dependent fashion [72]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AMD3100 (plerixafor, Mozobil®) was approved by the US FDA in December 2008 for hematopoietic progenitor cell mobilisation [41]. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antibodies | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Antibody Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Using their i-body platform, AdAlta have identified an anti-CXCR4 antibody (AD-114) that has proven effective as an anti-fibrotic agent in preclinical testing, in animal models of idiopathic pulmonary fibrosis (IPF) [18] and retinal fibrosis associated with age-related macular degeneration. AD-114 represents a novel mechanism of action for IPF, and a "first in class" therapy for this indication. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| CXCR4 is one of more than 20 distinct chemokine receptors expressed in human leukocytes. Chemokines primarily act to promote leukocyte chemotaxis to sites of inflammation. Due to its role in cancer cell homing and metastasis the CXCR4-CXCL12 axis is a potential target for cancer therapy [54,57,65-66]. This is exemplified by the development of ulocuplumab as an anti-cancer biologic therapy. Targeting the CXCR4-CXCL12 axis also offers the possibility of modulating the immune response in non-cancer indications [42,53,74]. |
| Immuno Disease Associations | ||||||||||
|
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gi/Go family | Calcium channel |
| References: 49,52 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clinically-Relevant Mutations and Pathophysiology Comments | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| CXCR4 haploinsufficiency promotes hematopoietic stem cell engraftment, suggesting a useful strategy to promote hematopoietic stem cell engraftment in transplantation [46]. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Biologically Significant Variants 
|
||||||||
|
||||||||
|
| General Comments |
|
CXCR4 is a receptor for stromal cell-derived factor-1 (SDF1; CXCL12) [9,51]. When co-expressed alongside CD4, CXCR4 is involved in HIV-1 infection. It is the major T cell-tropic coreceptor for HIV-1. In contrast, CCR5 serves as the principal macrophage-tropic coreceptor for viral entry. SDF1 inhibits HIV-1 infection by lymphocyte-tropic HIV-1 strains [9,51]. Cancer cells overexpress CXCR4 when compared to normal cells, and it is the most common chemokine receptor found on cancer cells. Its ligand CXCL12 is also found in the tumour micorenvironment [48]. Expression of CXCR4 correlates with cancer cell metastasis, angiogenesis, and tumour growth [10]. CXCR4 antagonists are predicted to exhibit anti-tumour activity via inhibition of CXCL12-CXCR4-mediated pro-survival signaling and chemotaxis [48,67]. |
References
1. Adlere I, Sun S, Zarca A, Roumen L, Gozelle M, Viciano CP, Caspar B, Arimont M, Bebelman JP, Briddon SJ et al.. (2019) Structure-based exploration and pharmacological evaluation of N-substituted piperidin-4-yl-methanamine CXCR4 chemokine receptor antagonists. Eur J Med Chem, 162: 631-649. DOI: 10.1016/j.ejmech.2018.10.060 [PMID:30476826]
2. Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O'Connor MJ, Doms RW, González-Scarano F. (1999) Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol, 73 (1): 205-13. [PMID:9847323]
3. Anton PA, Elliott J, Poles MA, McGowan IM, Matud J, Hultin LE, Grovit-Ferbas K, Mackay CR, Chen ISY, Giorgi JV. (2000) Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS, 14 (12): 1761-5. [PMID:10985313]
4. Ayehunie S, Garcia-Zepeda EA, Hoxie JA, Horuk R, Kupper TS, Luster AD, Ruprecht RM. (1997) Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood, 90 (4): 1379-86. [PMID:9269754]
5. Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O et al.. (2005) WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood, 105 (6): 2449-57. [PMID:15536153]
6. Banisadr G, Dicou E, Berbar T, Rostène W, Lombet A, Haour F. (2000) Characterization and visualization of [125I] stromal cell-derived factor-1alpha binding to CXCR4 receptors in rat brain and human neuroblastoma cells. J Neuroimmunol, 110 (1-2): 151-60. [PMID:11024545]
7. Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. (2005) The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci, 25 (16): 3995-4003. [PMID:15843601]
8. Berson JF, Long D, Doranz BJ, Rucker J, Jirik FR, Doms RW. (1996) A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol, 70 (9): 6288-95. [PMID:8709256]
9. Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature, 382 (6594): 829-33. [PMID:8752280]
10. Burger JA, Kipps TJ. (2006) CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood, 107 (5): 1761-7. [PMID:16269611]
11. Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. (1997) Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J, 16 (23): 6996-7007. [PMID:9384579]
12. Deichmann M, Kronenwett R, Haas R. (1997) Expression of the human immunodeficiency virus type-1 coreceptors CXCR-4 (fusin, LESTR) and CKR-5 in CD34+ hematopoietic progenitor cells. Blood, 89 (10): 3522-8. [PMID:9160656]
13. Delézay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. (1997) Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS, 11 (11): 1311-8. [PMID:9302439]
14. Di Salvo J, Koch GE, Johnson KE, Blake AD, Daugherty BL, DeMartino JA, Sirotina-Meisher A, Liu Y, Springer MS, Cascieri MA et al.. (2000) The CXCR4 agonist ligand stromal derived factor-1 maintains high affinity for receptors in both Galpha(i)-coupled and uncoupled states. Eur J Pharmacol, 409 (2): 143-54. [PMID:11104827]
15. Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, Heveker N, Volkman BF, Dwinell MB. (2011) Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci USA, 108 (43): 17655-60. [PMID:21990345]
16. Feng Y, Broder CC, Kennedy PE, Berger EA. (1996) HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science, 272 (5263): 872-7. [PMID:8629022]
17. Flynn G, Maru S, Loughlin J, Romero IA, Male D. (2003) Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol, 136 (1-2): 84-93. [PMID:12620646]
18. Foley M, Pow A, Griffiths K, Cobb S, Viduka K. (2016) Cxcr4 binding molecules. Patent number: WO2016109872. Assignee: Adalta Pty Ltd. Priority date: 09/01/2015. Publication date: 14/07/2016.
19. Fricker SP, Anastassov V, Cox J, Darkes MC, Grujic O, Idzan SR, Labrecque J, Lau G, Mosi RM, Nelson KL et al.. (2006) Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol, 72 (5): 588-96. [PMID:16815309]
20. Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. (2004) Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J Leukoc Biol, 76 (1): 185-94. [PMID:15075362]
21. Gupta SK, Pillarisetti K. (1999) Cutting edge: CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant. J Immunol, 163 (5): 2368-72. [PMID:10452968]
22. Habasque C, Aubry F, Jégou B, Samson M. (2002) Study of the HIV-1 receptors CD4, CXCR4, CCR5 and CCR3 in the human and rat testis. Mol Hum Reprod, 8 (5): 419-25. [PMID:11994538]
23. Heesen M, Berman MA, Benson JD, Gerard C, Dorf ME. (1996) Cloning of the mouse fusin gene, homologue to a human HIV-1 co-factor. J Immunol, 157 (12): 5455-60. [PMID:8955194]
24. Heesen M, Berman MA, Höpken UE, Gerard NP, Dorf ME. (1997) Alternate splicing of mouse fusin/CXC chemokine receptor-4: stromal cell-derived factor-1alpha is a ligand for both CXC chemokine receptor-4 isoforms. J Immunol, 158 (8): 3561-4. [PMID:9103415]
25. Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. (2003) Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet, 34 (1): 70-4. [PMID:12692554]
26. Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R. (1998) Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol, 160 (2): 877-83. [PMID:9551924]
27. Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells, 24 (4): 1030-41. [PMID:16253981]
28. Huber TB, Reinhardt HC, Exner M, Burger JA, Kerjaschki D, Saleem MA, Pavenstädt H. (2002) Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol, 168 (12): 6244-52. [PMID:12055238]
29. Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C, Patterson CJ, Sheehy P et al.. (2014) The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood, 123 (11): 1637-46. [PMID:24366360]
30. Iikura M, Miyamasu M, Yamaguchi M, Kawasaki H, Matsushima K, Kitaura M, Morita Y, Yoshie O, Yamamoto K, Hirai K. (2001) Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol, 70: 113-120. [PMID:11435493]
31. Inngjerdingen M, Damaj B, Maghazachi AA. (2001) Expression and regulation of chemokine receptors in human natural killer cells. Blood, 97 (2): 367-75. [PMID:11154210]
32. Jiang X, Lu L, Li J, Jiang J, Zhang J, Zhou S, Wen H, Cai H, Luo X, Li Z et al.. (2024) Synthetically Feasible De Novo Molecular Design of Leads Based on a Reinforcement Learning Model: AI-Assisted Discovery of an Anti-IBD Lead Targeting CXCR4. J Med Chem, 67 (12): 10057-10075. [PMID:38863440]
33. Jiang Y, Salafranca MN, Adhikari S, Xia Y, Feng L, Sonntag MK, deFiebre CM, Pennell NA, Streit WJ, Harrison JK. (1998) Chemokine receptor expression in cultured glia and rat experimental allergic encephalomyelitis. J Neuroimmunol, 86 (1): 1-12. [PMID:9655467]
34. Jordan NJ, Kolios G, Abbot SE, Sinai MA, Thompson DA, Petraki K, Westwick J. (1999) Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest, 104 (8): 1061-9. [PMID:10525044]
35. Karpova D, Bräuninger S, Wiercinska E, Krämer A, Stock B, Graff J, Martin H, Wach A, Escot C, Douglas G et al.. (2017) Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers-results of a dose escalation trial. J Transl Med, 15 (1): 2. [PMID:28049490]
36. Kitchen SG, Zack JA. (1997) CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol, 71 (9): 6928-34. [PMID:9261420]
37. Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Lüttichau HR, Gerstoft J, Clapham PR et al.. (1997) A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science, 277 (5332): 1656-9. [PMID:9287217]
38. Kowalska MA, Ratajczak J, Hoxie J, Brass LF, Gewirtz A, Poncz M, Ratajczak MZ. (1999) Megakaryocyte precursors, megakaryocytes and platelets express the HIV co-receptor CXCR4 on their surface: determination of response to stromal-derived factor-1 by megakaryocytes and platelets. Br J Haematol, 104 (2): 220-9. [PMID:10050701]
39. Kuhne M, Brams P,Tanamachi DM,Korman AJ, Cardarelli JM. (2013) Human monoclonal antibodies that bind CXCR4. Patent number: US8450464 B2. Assignee: Medarex, Inc.. Priority date: 02/10/2006. Publication date: 28/05/2013.
40. Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. (2008) CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood, 112 (1): 34-44. [PMID:18436740]
41. Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, Bridger G, Dunbar CE, Hematti P. (2006) AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood, 107 (9): 3772-8. [PMID:16439684]
42. Liu X, Mao J, Han C, Peng S, Li C, Jin T, Fan C, Shan Z, Teng W. (2016) CXCR4 antagonist AMD3100 ameliorates thyroid damage in autoimmune thyroiditis in NOD.H‑2h⁴ mice. Mol Med Rep, 13 (4): 3604-12. [PMID:26935473]
43. Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. (1994) Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem, 269 (1): 232-7. [PMID:8276799]
44. Loetscher P, Gong JH, Dewald B, Baggiolini M, Clark-Lewis I. (1998) N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem, 273 (35): 22279-83. [PMID:9712844]
45. Lu M, Grove EA, Miller RJ. (2002) Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA, 99 (10): 7090-5. [PMID:11983855]
46. McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N et al.. (2015) Chromothriptic cure of WHIM syndrome. Cell, 160 (4): 686-99. [PMID:25662009]
47. Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. (1998) Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA, 95 (24): 14500-5. [PMID:9826729]
48. Miller EJ, Jecs E, Truax VM, Katzman BM, Tahirovic YA, Wilson RJ, Kuo KM, Kim MB, Nguyen HH, Saindane MT et al.. (2018) Discovery of Tetrahydroisoquinoline-Containing CXCR4 Antagonists with Improved in Vitro ADMET Properties. J Med Chem, 61 (3): 946-979. [PMID:29350534]
49. Moepps B, Frodl R, Rodewald HR, Baggiolini M, Gierschik P. (1997) Two murine homologues of the human chemokine receptor CXCR4 mediating stromal cell-derived factor 1alpha activation of Gi2 are differentially expressed in vivo. Eur J Immunol, 27 (8): 2102-12. [PMID:9295051]
50. Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O'Brien W, Raz E, Littman D, Wylie C et al.. (2003) The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development, 130 (18): 4279-86. [PMID:12900445]
51. Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF et al.. (1996) The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature, 382 (6594): 833-5. [PMID:8752281]
52. Oh SB, Endoh T, Simen AA, Ren D, Miller RJ. (2002) Regulation of calcium currents by chemokines and their receptors. J Neuroimmunol, 123 (1-2): 66-75. [PMID:11880151]
53. Pozzobon T, Goldoni G, Viola A, Molon B. (2016) CXCR4 signaling in health and disease. Immunol Lett, 177: 6-15. [PMID:27363619]
54. Randhawa S, Cho BS, Ghosh D, Sivina M, Koehrer S, Müschen M, Peled A, Davis RE, Konopleva M, Burger JA. (2016) Effects of pharmacological and genetic disruption of CXCR4 chemokine receptor function in B-cell acute lymphoblastic leukaemia. Br J Haematol, 174 (3): 425-36. [PMID:27071778]
55. Rueda P, Balabanian K, Lagane B, Staropoli I, Chow K, Levoye A, Laguri C, Sadir R, Delaunay T, Izquierdo E et al.. (2008) The CXCL12gamma chemokine displays unprecedented structural and functional properties that make it a paradigm of chemoattractant proteins. PLoS ONE, 3 (7): e2543. [PMID:18648536]
56. Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. (1999) Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol, 154 (4): 1125-35. [PMID:10233851]
57. Scala S. (2015) Molecular Pathways: Targeting the CXCR4-CXCL12 Axis--Untapped Potential in the Tumor Microenvironment. Clin Cancer Res, 21 (19): 4278-85. [PMID:26199389]
58. Sehgal A, Ricks S, Boynton AL, Warrick J, Murphy GP. (1998) Molecular characterization of CXCR-4: a potential brain tumor-associated gene. J Surg Oncol, 69 (4): 239-48. [PMID:9881942]
59. Skerlj RT, Bridger GJ, Kaller A, McEachern EJ, Crawford JB, Zhou Y, Atsma B, Langille J, Nan S, Veale D et al.. (2010) Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem, 53 (8): 3376-88. [PMID:20297846]
60. Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. (2003) CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci, 23 (12): 5123-30. [PMID:12832536]
61. Sun Y, Cheng Z, Ma L, Pei G. (2002) Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem, 277 (51): 49212-9. [PMID:12370187]
62. Tamamura H, Hiramatsu K, Mizumoto M, Ueda S, Kusano S, Terakubo S, Akamatsu M, Yamamoto N, Trent JO, Wang Z et al.. (2003) Enhancement of the T140-based pharmacophores leads to the development of more potent and bio-stable CXCR4 antagonists. Org Biomol Chem, 1 (21): 3663-9. [PMID:14649897]
63. Tamamura H, Xu Y, Hattori T, Zhang X, Arakaki R, Kanbara K, Omagari A, Otaka A, Ibuka T, Yamamoto N et al.. (1998) A low-molecular-weight inhibitor against the chemokine receptor CXCR4: a strong anti-HIV peptide T140. Biochem Biophys Res Commun, 253 (3): 877-82. [PMID:9918823]
64. Thoma G, Streiff MB, Kovarik J, Glickman F, Wagner T, Beerli C, Zerwes HG. (2008) Orally bioavailable isothioureas block function of the chemokine receptor CXCR4 in vitro and in vivo. J Med Chem, 51 (24): 7915-20. [PMID:19053768]
65. Walenkamp AME, Lapa C, Herrmann K, Wester HJ. (2017) CXCR4 Ligands: The Next Big Hit?. J Nucl Med, 58 (Suppl 2): 77S-82S. [PMID:28864616]
66. Wang Z, Sun J, Feng Y, Tian X, Wang B, Zhou Y. (2016) Oncogenic roles and drug target of CXCR4/CXCL12 axis in lung cancer and cancer stem cell. Tumour Biol, 37 (7): 8515-28. [PMID:27079871]
67. Wilson RJ, Jecs E, Miller EJ, Nguyen HH, Tahirovic YA, Truax VM, Kim MB, Kuo KM, Wang T, Sum CS et al.. (2018) Synthesis and SAR of 1,2,3,4-Tetrahydroisoquinoline-Based CXCR4 Antagonists. ACS Med Chem Lett, 9 (1): 17-22. [PMID:29348805]
68. Wong ML, Xin WW, Duman RS. (1996) Rat LCR1: cloning and cellular distribution of a putative chemokine receptor in brain. Mol Psychiatry, 1 (2): 133-40. [PMID:9118323]
69. Wong RS, Bodart V, Metz M, Labrecque J, Bridger G, Fricker SP. (2008) Comparison of the potential multiple binding modes of bicyclam, monocylam, and noncyclam small-molecule CXC chemokine receptor 4 inhibitors. Mol Pharmacol, 74 (6): 1485-95. [PMID:18768385]
70. Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC et al.. (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science, 330 (6007): 1066-71. [PMID:20929726]
71. Wu KJ, Yu SJ, Shia KS, Wu CH, Song JS, Kuan HH, Yeh KC, Chen CT, Bae E, Wang Y. (2017) A Novel CXCR4 Antagonist CX549 Induces Neuroprotection in Stroke Brain. Cell Transplant, 26 (4): 571-583. [PMID:27938478]
72. Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, Su EW, Wang J. (2006) Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene, 374: 174-9. [PMID:16626895]
73. Zamarchi R, Allavena P, Borsetti A, Stievano L, Tosello V, Marcato N, Esposito G, Roni V, Paganin C, Bianchi G et al.. (2002) Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes. Clin Exp Immunol, 127 (2): 321-30. [PMID:11876757]
74. Zhang H, Kang D, Huang B, Liu N, Zhao F, Zhan P, Liu X. (2016) Discovery of non-peptide small molecular CXCR4 antagonists as anti-HIV agents: Recent advances and future opportunities. Eur J Med Chem, 114: 65-78. [PMID:26974376]
75. Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A, Pei G, Manfredi JP, Fujii N, Broach JR, Peiper SC. (2002) A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem, 277: 24515-24521. [PMID:11923301]
76. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. (1998) Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature, 393 (6685): 595-9. [PMID:9634238]

 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb













