GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
transforming growth factor beta receptor 1
 Target has curated data in GtoImmuPdb
Target has curated data in GtoImmuPdb
Target id: 1788
Nomenclature: transforming growth factor beta receptor 1
Abbreviated Name: TGFBR1
Contents:
- Quaternary Structure
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Enzyme Reaction
- Natural/Endogenous Ligands
- Inhibitors
- Agonists
- Large-scale Ligand Screening Data
- Immunopharmacology Comments
- Clinically-Relevant Mutations and Pathophysiology
- References
- How to cite this page
| Quaternary Structure: Complexes |
| Transforming growth factor β receptor |
| Growth/differentiation factor receptors |
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 503 | 9q22.33 | TGFBR1 | transforming growth factor beta receptor 1 | |
| Mouse | 1 | 503 | 4 26.02 cM | Tgfbr1 | transforming growth factor, beta receptor I | |
| Rat | 1 | 501 | 5q22 | Tgfbr1 | transforming growth factor, beta receptor 1 | |
Database Links  |
|
| Alphafold | P36897 (Hs), Q64729 (Mm), P80204 (Rn) |
| BRENDA | 2.7.11.30 |
| ChEMBL Target | CHEMBL4439 (Hs), CHEMBL2021750 (Mm), CHEMBL4523265 (Rn) |
| Ensembl Gene | ENSG00000106799 (Hs), ENSMUSG00000007613 (Mm), ENSRNOG00000007036 (Rn) |
| Entrez Gene | 7046 (Hs), 21812 (Mm) |
| Human Protein Atlas | ENSG00000106799 (Hs) |
| KEGG Enzyme | 2.7.11.30 |
| KEGG Gene | hsa:7046 (Hs), mmu:21812 (Mm) |
| OMIM | 190181 (Hs) |
| Orphanet | ORPHA120065 (Hs) |
| Pharos | P36897 (Hs) |
| RefSeq Nucleotide | NM_001130916 (Hs), NM_009370 (Mm), NM_012775 (Rn) |
| RefSeq Protein | NP_001124388 (Hs), NP_033396 (Mm), NP_036907 (Rn) |
| SynPHARM |
81808 (in complex with SB-431542) 80583 (in complex with TGF-beta RI kinase inhibitor) |
| UniProtKB | P36897 (Hs), Q64729 (Mm), P80204 (Rn) |
| Wikipedia | TGFBR1 (Hs) |
Selected 3D Structures  |
|||||||||||||
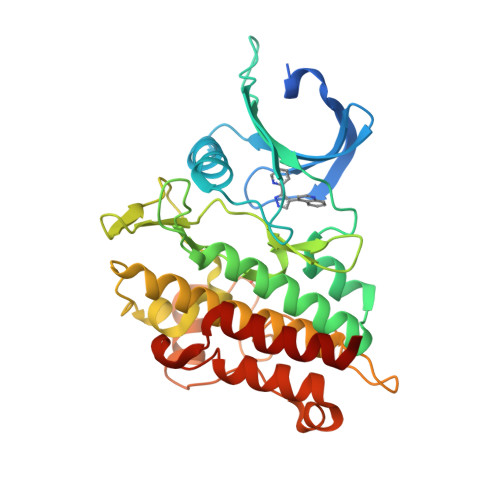
|
|
||||||||||||
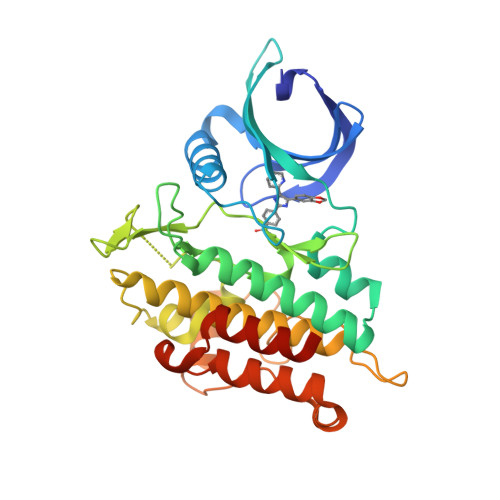
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Natural/Endogenous Ligands  |
| growth/differentiation factor-11 {Sp: Human} |
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 7,22 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: TGFBR1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 2,9 |
 
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: TGFBR1/ALK5(TGFBR1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| The role of TGFBR1 in immuno-oncology is reviewed in [1]. |
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
References
1. Adams JL, Smothers J, Srinivasan R, Hoos A. (2015) Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov, 14 (9): 603-22. [PMID:26228631]
2. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
3. Arista L, Babu S, Bian J, Cui K, Dillon MP, Lattmann R, Li J, Liao L, Lizos D, Ramos R et al.. (2021) Aminopyridine derivatives and their use as selective alk-2 inhibitors. Patent number: US20210155606A1. Assignee: Novartis AG. Priority date: 20/07/2016. Publication date: 27/05/2021.
4. Artal RB, Casal BP, Laria JCP. (2021) Benzylamide derivatives as inhibitors of transforming growth factor-beta receptor i/alk5. Patent number: WO2021105317A1. Assignee: Origo Biopharma, S.L.. Priority date: 27/11/2020. Publication date: 03/06/2021.
5. Bamborough P, Drewry D, Harper G, Smith GK, Schneider K. (2008) Assessment of chemical coverage of kinome space and its implications for kinase drug discovery. J Med Chem, 51 (24): 7898-914. [PMID:19035792]
6. Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A et al.. (2002) Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5). J Med Chem, 45 (5): 999-1001. [PMID:11855979]
7. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
8. Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR. (2013) Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorg Med Chem Lett, 23 (11): 3248-52. [PMID:23639540]
9. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
10. Jin CH, Krishnaiah M, Sreenu D, Subrahmanyam VB, Rao KS, Lee HJ, Park SJ, Park HJ, Lee K, Sheen YY et al.. (2014) Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-β type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem, 57 (10): 4213-38. [PMID:24786585]
11. Jin CH, Sreenu D, Krishnaiah M, Subrahmanyam VB, Rao KS, Nagendra Mohan AV, Park CY, Son JY, Son DH, Park HJ et al.. (2011) Synthesis and biological evaluation of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles as transforming growth factor-β type 1 receptor kinase inhibitors. Eur J Med Chem, 46 (9): 3917-25. [PMID:21696866]
12. Johnston CJC, Smyth DJ, Kodali RB, White MPJ, Harcus Y, Filbey KJ, Hewitson JP, Hinck CS, Ivens A, Kemter AM et al.. (2017) A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun, 8 (1): 1741. [PMID:29170498]
13. Lee JY, Ryu KH, Kim JS, Kim YH, Shin DCS, Lee BY, Kang SH, Lee HJ, Jung H, Shin YA et al.. (2018) 2-pyridyl substituted imidazoles as ALK5 and/or ALK4 inhibitors. Patent number: US10155763B2. Assignee: Tiumbio Co Ltd. Priority date: 13/07/2012. Publication date: 18/12/2018.
14. Li HY, Wang Y, Heap CR, King CH, Mundla SR, Voss M, Clawson DK, Yan L, Campbell RM, Anderson BD et al.. (2006) Dihydropyrrolopyrazole transforming growth factor-beta type I receptor kinase domain inhibitors: a novel benzimidazole series with selectivity versus transforming growth factor-beta type II receptor kinase and mixed lineage kinase-7. J Med Chem, 49 (6): 2138-42. [PMID:16539403]
15. Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ. (2008) LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther, 7 (4): 829-40. [PMID:18413796]
16. Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. (2014) Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem, 57 (19): 7900-15. [PMID:25101911]
17. Ogunjimi AA, Zeqiraj E, Ceccarelli DF, Sicheri F, Wrana JL, David L. (2012) Structural basis for specificity of TGFβ family receptor small molecule inhibitors. Cell Signal, 24 (2): 476-83. [PMID:21983015]
18. Raboisson P, Lenz O, Lin TI, Surleraux D, Chakravarty S, Scholliers A, Vermeiren K, Delouvroy F, Verbinnen T, Simmen K. (2007) Evaluation of the anti-hepatitis C virus effect of novel potent, selective, and orally bioavailable JNK and VEGFR kinase inhibitors. Bioorg Med Chem Lett, 17 (7): 1843-9. [PMID:17289388]
19. Sawyer JS, Beight DW, Britt KS, Anderson BD, Campbell RM, Goodson T, Herron DK, Li HY, McMillen WT, Mort N et al.. (2004) Synthesis and activity of new aryl- and heteroaryl-substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. Bioorg Med Chem Lett, 14 (13): 3581-4. [PMID:15177479]
20. Tatake RJ, O'Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D, Kugler Jr SZ, Kashem MA, Kaplita P, Snow RJ. (2008) Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem Biophys Res Commun, 377 (1): 120-5. [PMID:18834865]
21. Velaparthi U, Darne CP, Warrier J, Liu P, Rahaman H, Augustine-Rauch K, Parrish K, Yang Z, Swanson J, Brown J et al.. (2020) Discovery of BMS-986260, a Potent, Selective, and Orally Bioavailable TGFβR1 Inhibitor as an Immuno-oncology Agent. ACS Med Chem Lett, 11 (2): 172-178. DOI: 10.1021/acsmedchemlett.9b00552 [PMID:32071685]
22. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
23. Yingling JM, McMillen WT, Yan L, Huang H, Sawyer JS, Graff J, Clawson DK, Britt KS, Anderson BD, Beight DW et al.. (2018) Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget, 9 (6): 6659-6677. [PMID:29467918]
How to cite this page
Type I receptor serine/threonine kinases: transforming growth factor beta receptor 1. Last modified on 08/04/2025. Accessed on 28/01/2026. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1788.











