|
Synonyms: GDC-0032 | GDC0032
Compound class:
Synthetic organic
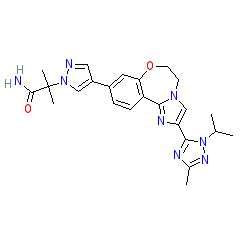
Comment: Taselisib is a potent and orally bioavailable kinase inhibitor which inhibits the PI3Kα, γ and δ isoforms but not the β isoform. It is compound 11l in [3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Braun M-G, Hanan E, Staben ST, Heald RA, Macleod C, Elliott R. (2017)
Benzoxazepin oxazolidinone compounds and methods of use. Patent number: US20170015678. Assignee: Genentech, Inc.. Priority date: 02/07/2015. Publication date: 19/01/2017. |
|
2. Lopez S, Schwab CL, Cocco E, Bellone S, Bonazzoli E, English DP, Schwartz PE, Rutherford T, Angioli R, Santin AD. (2014)
Taselisib, a selective inhibitor of PIK3CA, is highly effective on PIK3CA-mutated and HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol, 135 (2): 312-7. [PMID:25172762] |
|
3. Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, Bull R, Do S, Dotson J, Dudley D et al.. (2013)
Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem, 56 (11): 4597-610. [PMID:23662903] |