GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
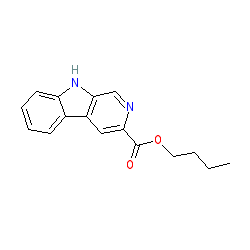
Synonyms: n-butyl-β-carboline-3-carboxylate | n-butyl-beta-carboline-3-carboxylate
Compound class:
Synthetic organic
Comment: This compound is represented on ChEMBL by the entry CHEMBL148296 which shows the double bonds in different positions.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Mangin JM, Nguyen L, Gougnard C, Hans G, Rogister B, Belachew S, Moonen G, Legendre P, Rigo JM. (2005)
Developmental regulation of beta-carboline-induced inhibition of glycine-evoked responses depends on glycine receptor beta subunit expression. Mol Pharmacol, 67 (5): 1783-96. [PMID:15722459] |