|
Compound class:
Synthetic organic
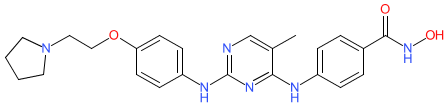
Comment: This compund is a multitarget agent that is designed to inhibit the BRD4-LIFR-JAK1-STAT3 signalling pathway in tumour cells in which this pathway has been implicated in resistance to HDAC inhibitors [3]. The design of 25ap was based on fedratinib, which has dual JAK/BRD4 inhibitory actions [1-2]. Functionally, 25ap acts as a potent HDAC/JAK/BRD4 triple inhibitor that simultaneously elevates acetylation of histone and α-tubulin, reduces STAT3 phosphorylation and downregulates transcription of leukemia inhibitory factor receptor (LIFR), MCL1 apoptosis regulator (MCL-1), and c-Myc oncogene in breast cancer cells, which mediate antiproliferative, pro-apoptotic and anti-clonogenic effects in vitro. In vivo antitumour efficacy was demonstrated in a MDA-MB-231 breast cancer xenograft model.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Ciceri P, Müller S, O'Mahony A, Fedorov O, Filippakopoulos P, Hunt JP, Lasater EA, Pallares G, Picaud S, Wells C et al.. (2014)
Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat Chem Biol, 10 (4): 305-12. [PMID:24584101] |
|
2. Ember SW, Zhu JY, Olesen SH, Martin MP, Becker A, Berndt N, Georg GI, Schönbrunn E. (2014)
Acetyl-lysine binding site of bromodomain-containing protein 4 (BRD4) interacts with diverse kinase inhibitors. ACS Chem Biol, 9 (5): 1160-71. [PMID:24568369] |
|
3. Zhao C, Zhang Y, Zhang J, Li S, Liu M, Geng Y, Liu F, Chai Q, Meng H, Li M et al.. (2023)
Discovery of Novel Fedratinib-Based HDAC/JAK/BRD4 Triple Inhibitors with Remarkable Antitumor Activity against Triple Negative Breast Cancer. J Med Chem, 66 (20): 14150-14174. [PMID:37796543] |