|
Synonyms: CIN-107 | CIN107 | RO6836191
Compound class:
Synthetic organic
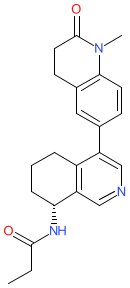
Comment: Baxdrostat (CIN-107, formerly RO6836191 [1]) is a selective, oral aldosterone synthase (CYP11B2) inhibitor. It inhibits aldosterone biosynthesis but does not alter plasma cortisol, which demonstrates its selectivity for CYP11B2 [3]. Baxdrostat is being developed by CinCor Pharma as a potential intervention for treatment resistant hypertension.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bogman K, Schwab D, Delporte ML, Palermo G, Amrein K, Mohr S, De Vera Mudry MC, Brown MJ, Ferber P. (2017)
Preclinical and Early Clinical Profile of a Highly Selective and Potent Oral Inhibitor of Aldosterone Synthase (CYP11B2). Hypertension, 69 (1): 189-196. [PMID:27872236] |
|
2. Forzano I, Mone P, Varzideh F, Jankauskas SS, Kansakar U, De Luca A, Santulli G. (2022)
The selective aldosterone synthase inhibitor Baxdrostat significantly lowers blood pressure in patients with resistant hypertension. Front Endocrinol (Lausanne), 13: 1097968. [PMID:36568122] |
|
3. Freeman MW, Bond M, Murphy B, Hui J, Isaacsohn J. (2023)
Results from a phase 1, randomized, double-blind, multiple ascending dose study characterizing the pharmacokinetics and demonstrating the safety and selectivity of the aldosterone synthase inhibitor baxdrostat in healthy volunteers. Hypertens Res, 46 (1): 108-118. [PMID:36266539] |