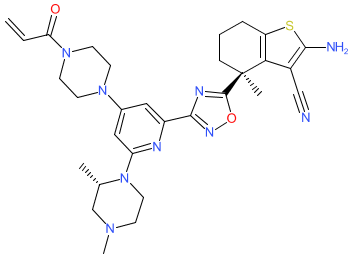
|
Synonyms: BI 0474 | BI0474 | compound 23 [PMID: 36300829]
Compound class:
Synthetic organic
Comment: BI-0474 is an inhibitor of the oncogenic protein KRASG12C [1]. It was designed to target KRASG12C-driven tumours but it is not orally bioactive. Boehringer have an alternative orally bioavailable analogue (BI 1823911) from the same series as BI-0474, in clinical development (NCT04973163), but its structure has not been disclosed (Nov. 2022).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bröker J, Waterson AG, Smethurst C, Kessler D, Böttcher J, Mayer M, Gmaschitz G, Phan J, Little A, Abbott JR et al.. (2022)
Fragment Optimization of Reversible Binding to the Switch II Pocket on KRAS Leads to a Potent, In Vivo Active KRASG12C Inhibitor. J Med Chem, 65 (21): 14614-14629. [PMID:36300829] |