|
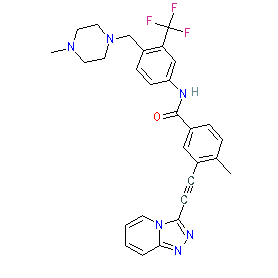
Synonyms: compound 1 (Table 1) [WO2012173521] | PF-114 | PF114
Compound class:
Synthetic organic
Comment: Vamotinib (PF-114) is a third generation BCR-ABL kinase inhibitor [2-3]. It was designed to target wild-type resistance mutations in BCR-ABL (including the T315I gatekeeper mutation) in order to overcome drug resistance in Philadelphia chromosome positive (Ph+) leukemias. The chemical strucure is claimed in patent WO2012173521 where it is the first compound listed in Table 1 of the patent [1]. Off-targets identified by kinase profile screening include ABL2/ARG, DDR1, DDR2, FMS, FRK/PTK5, LCK, LYN, LYNB, PDGFRα and RET which had <10% residual activity when exposed to 100 nM of inhibitor [3]. We matched PF-114's chemical structure to the INN 'vamotinib' that was released in the WHO's proposed INN list 127 (21 July 2022).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A Phase 1/2 clinical trial evaluating PF-114 in CML patients is underway- see NCT02885766. This study is focusing on leukemias that are resistsnt to second generation inhibitors or that are T315I gatekeeper mutation positive. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02885766 | Study to Evaluate Tolerability, Safety, Pharmacokinetics and Preliminary Efficacy of PF-114 for Oral Administration in Adults With Ph+ Chronic Myeloid Leukemia, Which is Resistant to the 2-nd Generation Bcr-Abl Inhibitors or Has T315I Mutation in the BCR-ABL Gene | Phase 1/Phase 2 Interventional | Fusion Pharma LLC | ||