|
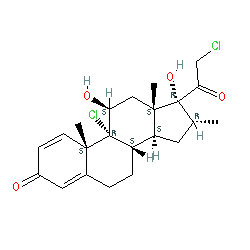
Synonyms: Elocon® (topical) | Nasonex®
mometasone is an approved drug (1987 (FDA))
Compound class:
Synthetic organic
Comment: Mometasone is a corticosteroid anti-inflammatory drug.

View more information in the IUPHAR Pharmacology Education Project: mometasone |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Mometasone is used intranasally in the management of asthma, allergic rhinitis and nasal polyposis, and topically for allergic skin disorders. Prescribed formulations contain mometasone furoate (PubChem CID 441336). A fixed-dose combination of indacaterol/glycopyrrolate/mometasone furoate (QVM149, Enerzair® Breezhaler®) has completed Phase 3 evaluation as a once-daily therapy for uncontrolled asthma [1]. Results confirmed non-inferiority to twice-daily salmeterol/fluticasone high-dose + tiotropium, and using a medium-dose had comparable efficacy but at a corresponding lower steroid dose. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03158311 | Study to Compare QVM149 and Free Triple Combination of Salmeterol/Fluticasone + Tiotropium | Phase 3 Interventional | Novartis | Based on results from this trial, this combination therapy is under regulatory review in multiple countries to gain clinical approval. | 1 |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com |