|
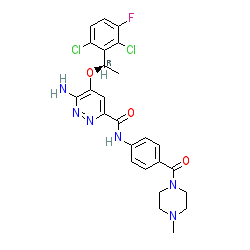
Synonyms: Example 18 [WO2012048259] [1] | X-396
Compound class:
Synthetic organic
Comment: X-396 is an orally available, investigational inhibitor of anaplastic lymphoma receptor tyrosine kinase (ALK). Lovly et al. (2011) report preclinical proof-of-concept findings for the utility of X-396 in ALK-driven cancers [2]
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| There are a number of ongoing clinical trials that are evaluating X-396 (ensartinib) in a variety of solid tumour types. The most advanced is a Phase 3 trial that is comparing ensartinib to crizotinib in ALK-rearranged NSCLC (NCT02767804). A Phase 2 study is underway to determine the efficacy of genetic screening as a route to personalising treatment for childhood non-Hodgkin lymphoma, in which ensartinib is one of the drugs available to test. Click here to link to ClinicalTrials.gov's full list of current ensartinib trials. In August 2020, Xcovery reported that ensartinib performed better than crizotinib as a firstline treatment in 290 patients with ALK-positive NSCLC, including in patients with brain metasteses. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02767804 | eXalt3: Study Comparing X-396 (Ensartinib) to Crizotinib in ALK Positive Non-Small Cell Lung Cancer (NSCLC) Patients | Phase 3 Interventional | Xcovery Holding Company, LLC | ||