|
Synonyms: JNJ-40346527 | JNJ40346527 | US8497376, 15
Compound class:
Synthetic organic
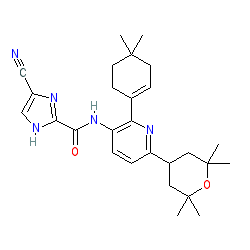
Comment: Edicotinib (PRV-6527; previously JNJ-40346527) was identified in a medicinal-chemistry lead optimization study to develop oral, selective CSF1R kinase inhibitors. The chemical structure is compound I-S in patent US20140045789 [2]. PubChem CID 25230468 is a tautomer which is also claimed in the patent.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| JNJ-40346527 has completed Phase 1/II clinical trial for relapsed or refractory Hodgkin's lymphoma (NCT01572519) and Phase 2 in patients with active rheumatoid arthritis (RA) who are not responding to current antirheumatic treatment (NCT01597739; results reported in [1]). Janssen terminated their development of JNJ-4034652 in 2014 due to a lack of efficacy. Subsequently, Provention Bio have tested PRV-6527 in Crohn's disease (see their Phase 2a PRINCE clinical trial NCT03854305; no results formally published as of October 2019). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01572519 | A Study of JNJ-40346527 in Patients With Relapsed or Refractory Hodgkin Lymphoma | Phase 1 Interventional | Janssen Research & Development, LLC | ||
| NCT01597739 | A Study of JNJ-40346527 in Patients With Active Rheumatoid Arthritis Despite Disease-modifying Antirheumatic Drug Therapy | Phase 2 Interventional | Janssen Research & Development, LLC | ||
| NCT03854305 | Phase 2a Study of PRV-6527 in Subjects With Moderately to Severely Active Crohn's Disease | Phase 2 Interventional | Provention Bio, Inc. | ||