|
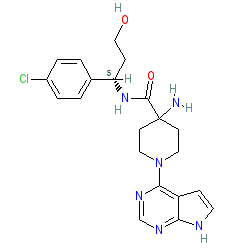
Synonyms: AZD 5363 | AZD-5363 | AZD5363 | cc-638 | Truqap®
capivasertib is an approved drug (FDA (2023))
Compound class:
Synthetic organic
Comment: Capivasertib (AZD5363) was developed as an inhibitor of the AKT serine/threonine protein kinases [1], and was investigated as a potential therapeutic for solid and haematological malignancies. This compound is now included in AstaZeneca's Open Innovation Pharmacology Toolbox.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Capivasertib was evaluated in clinical trials in patients with various types of advanced solid malignancies. Click here to link to ClinicalTrials.gov's full list of capivasertib trials. First approval was granted by the FDA in November 2023, under which capivasertib (with fulvestrant) was authorised to treat hormone receptor +ve, HER2 -ve locally advanced/metastatic breast cancer with ≥ PIK3CA/AKT1/PTEN genetic alterations (that promote AKT pathway activation). The FoundationOne®CDx assay must be used to confirm the presence of the specified genetic alterations. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04305496 | Capivasertib+Fulvestrant vs Placebo+Fulvestrant as Treatment for Locally Advanced (Inoperable) or Metastatic HR+/HER2- Breast Cancer | Phase 3 Interventional | AstraZeneca | 3 | |