|
Synonyms: 4SC-201 | BYK408740 | RAS2410
Compound class:
Synthetic organic
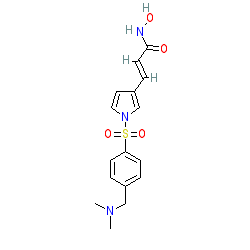
Comment: Resminostat is an HDAC inhibitor with selectivity for HDACs 1, 3 and 6, with significantly lower affinity for HDAC8 [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In 2011, the European Medicines Agency granted orphan designation to resminostat for the treatment of patients with Hodgkin's lymphoma. Click here to link to ClinicalTrials.gov's full list of resminostat studies. |
Mechanism Of Action and Pharmacodynamic Effects  |
| In Hodgkin's lymphoma, resminostat swittches on genes which suppress the division and growth of the tumour cells, thereby leading to a reduction in the growth and division of the cancerous cells. Resminostat modulates the expression/activity of apoptotisis-associated proteins such as Bim, Bax, Bcl-xL, and caspases 3, 8 and 9 [1]. |