GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
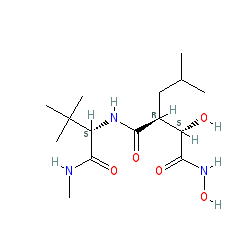
Synonyms: BB-2516 | BB2516
Compound class:
Synthetic organic
Comment: Marimastat (BB-2516) is a broad spectrum matrix metalloprotease (MMP) inhibitor. Its MMP inhibitory action has been applied to treating cancer [3,5]. Most recently marimastat has been reported to inhibit certain snake venom metalloproteases [2], with the implication that it could be repurposed as an antidote to snake venom poisoning [6].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT00261391 | Phase I Drug Trial for S/E of Marimastat in Disabling Malformations When no Other Options. | Phase 1 Interventional | Boston Children’s Hospital | ||
| NCT00003011 | Marimastat Following Chemotherapy in Treating Patients With Small Cell Lung Cancer | Phase 3 Interventional | Canadian Cancer Trials Group | ||
| NCT00002911 | Marimastat in Treating Patients With Stage III Non-small Cell Lung Cancer | Phase 3 Interventional | National Cancer Institute (NCI) | ||
| NCT00003010 | Marimastat or No Further Therapy in Treating Women With Metastatic Breast Cancer That Is Responding or Stable Following Chemotherapy | Phase 3 Interventional | Eastern Cooperative Oncology Group | In this study, marimastat did not prolong progression-free survival when used after first-line chemotherapy for metastatic breast cancer. | 4 |