|
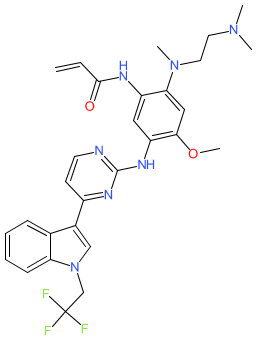
Synonyms: compound 4 [WO2019218987A1] [2] | D-0316 | D0316 | Surmana®
befotertinib is an approved drug (China NMPA (2023))
Compound class:
Synthetic organic
Comment: Befotertinib (D-0316) is an third generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor that was developed as a treatment for non-small cell lung cancer (NSCLC) [2].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Befotertinib was approved in China in May 2023, as a second-line treatment for advanced or metastatic, progessing EGFRT790M positive NSCLC [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03861156 | D-0316 in Patients With EGFR Positive Non Small Cell Lung Cancer | Phase 2 Interventional | InventisBio Co., Ltd | 4 | |
| NCT04206072 | D-0316 Versus Icotinib in Patients With Locally Advanced or Metastatic EGFR Sensitising Mutation Positive NSCLC | Phase 2/Phase 3 Interventional | Betta Pharmaceuticals Co., Ltd. | 5 | |