|
Synonyms: ALK2-IN-1 | BLU-782 | BLU782 | IPN-60130 | IPN60130
Compound class:
Synthetic organic
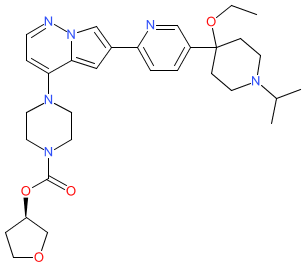
Comment: The chemical structure for fidrisertib was obtained from the WHO proposed INN list 126 (Jan 2022). This matches to the company code BLU-782 via PubChem. The compound is the subject of Blueprint Medicines' patent WO2021030386A1 [2], in which it is described as an activin receptor-like kinase (ALK) inhibitor, with preferred selectivity for ALK2 (ACVR1) and mutant forms of ALK2.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03858075 | Safety, Tolerability, Pharmacokinetics, and Food Effect of BLU-782 in Healthy Adults | Phase 1 Interventional | Blueprint Medicines Corporation | ||
| NCT05039515 | Study to Assess the Efficacy and Safety of 2 Dosage Regimens of Oral IPN60130 for the Treatment of Fibrodysplasia Ossificans Progressiva (FOP). | Phase 2 Interventional | Ipsen | ||