|
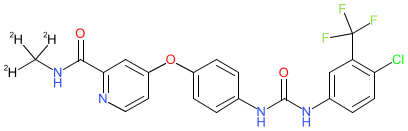
Synonyms: CM-4307 | CM4307 | Sorafenib-d3 | Zepsun®
Compound class:
Synthetic organic
Comment: Donafenib (CM-4307) is a deuterated derivative of the approved multikinase inhibitor sorafenib. Like sorafenib, it is orally active. Deuteration improves the stability of drug molecules thus extending half-life and increasing systemic exposure. This means that the effective drug dose may be shifted downward, with an associated improvement in tolerability. The medical formulation is donafenib tosylate.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Donafenib (CM-4307) was progressed to clinical evaluations to determine its efficacy against advanced solid tumours. It exhibited superior overall survival results compared to sorafenib in a direct comparison study in patients with unresectable/metastatic hepatocellular carcinoma [3]. Donafenib was granted its first international approval in China (June 2021) [1]. This approval indicated donafenib as a first-line systemic therapy for unresectable hepatocellular carcinoma. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05138159 | A Study of Donafenib Plus S-1 in Treating Patients With Metastatic Pancreatic Cancer After Chemotherapy With Nab-paclitaxel Plus Gemcitabine Regimen | Phase 2 Interventional | Fudan University | ||
| NCT02645981 | Efficacy and Safety of Donafenib in Patients With Advanced Hepatocellular Carcinoma | Phase 2/Phase 3 Interventional | Suzhou Zelgen Biopharmaceuticals Co.,Ltd | ||
| NCT02229071 | Safety and Efficacy of Donafenib in Patients With Advanced Hepatocellular Carcinoma | Phase 1/Phase 2 Interventional | Suzhou Zelgen Biopharmaceuticals Co.,Ltd | 2 | |