|
Synonyms: compound 41 [PMID: 32073845] | Fabhalta® | LNP-023 | LNP023
iptacopan is an approved drug (FDA (2023))
Compound class:
Synthetic organic
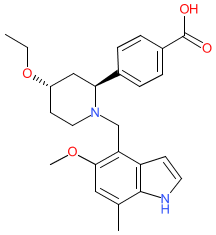
Comment: Iptacopan (LNP023) is a small molecuile, orally bioavailable complement factor B inhibitor [1,3]. Factor B is a serine protease, that is a component of the alternative pathway (AP) of the complement system. The AP acts as an amplification loop of complement activation and contributes to various human diseases. Inhibition of factor B is being expolited as a mechanism to treat complement-mediated conditions such as C3 glomerulopathy (glomerulonephritis, including IgA nephropathy), paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03373461 | Study of Safety and Efficacy of LNP023 in Patients With Kidney Disease Caused by Inflammation | Phase 2 Interventional | Novartis | ||
| NCT03955445 | OL Extension Study of LNP023 in C3G | Phase 2 Interventional | Novartis | ||
| NCT03439839 | Study of Safety, Efficacy, Tolerability, Pharmacokinetics and Pharmacodynamics of LNP023 in in Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH) | Phase 2 Interventional | Novartis | 2 | |