|
Synonyms: example 3 [WO2015048662A2] | ICP-022 | ICP022
orelabrutinib is an approved drug (China (2020))
Compound class:
Synthetic organic
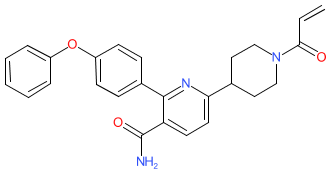
Comment: The chemical structure that was submitted to the WHO for the INN orelabrutinib, is identical to the structure of example 3 that is claimed as a BTK inhibitor in patent WO2015048662A2 [2]. The inhibitors in WO2015048662A2 are claimed for their potential to treat cancers [1] and inflammatory/autoimmune diseases that are mediated, at least in part, by Bruton's tyrosine kinase (BTK). Innocare Pharma have declared the ICP-022>orelabrutinib association on their company news webpage. Orelabrutinib is an orally administered, potent, irreversible and selective BTK-inhibitor.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Orelabrutinib (ICP-022) has advanced to Phase 2 evaluation in patients with relapsed/refractory marginal zone lymphoma (MZL). It is also being investigated in other B cell malignancies. Click here to link to ClinicalTrials.gov's full list of ICP-022 studies. China granted approval in December 2020, for the treatment of previously treated mantle cell lymphoma (MCL) or chronic lymphocytic leukaemia (CLL)/small lymphocytic lymphoma (SLL) [3]. FDA orphan designation for MCL was also issued in December 2020. In February 2021 the Chinese approval was expanded to include use as a first-line MCL therapy in combination with R-CHOP chemotherapy. In July 2021 it was revealed that Biogen will be partnering with InnoCare to investigate orelabrutinib's potential in relapsing/remitting multiple sclerosis. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04438005 | A Study of ICP-022 in Patients With R/R DLBCL | Phase 2 Interventional | Beijing InnoCare Pharma Tech Co., Ltd. | ||
| NCT03494179 | A Study to Evaluate ICP-022 in Patients With R/R Mantle Cell Lymphoma (MCL) | Phase 1/Phase 2 Interventional | Beijing InnoCare Pharma Tech Co., Ltd. | ||
| NCT04578613 | ICP-022 Versus Chlorambucil Combined With Rituximab in the Treatment of Untreated CLL/SLL | Phase 3 Interventional | Beijing InnoCare Pharma Tech Co., Ltd. | ||
| NCT04305197 | A Study of ICP-022 in Patients With Systemic Lupus Erythematosus (SLE) | Phase 1/Phase 2 Interventional | Beijing InnoCare Pharma Tech Co., Ltd. | ||
| NCT04831658 | A Prospective Clinical Study of BTK Inhibitor, PD-1 and Formustine in the First-line Treatment of Primary Central Nervous System Lymphoma | Phase 4 Interventional | Zhengzhou University | ||
| NCT04711148 | A Phase 2 Study of Orelabrutinib in Patients With Relapsing-Remitting Multiple Sclerosis | Phase 2 Interventional | Beijing InnoCare Pharma Tech Co., Ltd. | ||