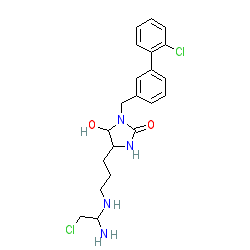
|
Compound class:
Synthetic organic
Comment: Compound 14b is reported as an irreversible and selective inhibitor of protein arginine deiminase type III (PADI3) [1].
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| As compound 14b is an irreversible inhibitor the authors provide inhibition values as rate constants (kinact/KI) as a measure of inhibitory activity. NB click on the down arrow at the end of the row in the table below to reveal the rate constant. |
| Selectivity at enzymes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||