GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
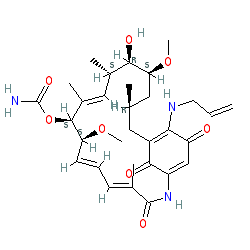
Synonyms: 17-AAG | 17-allylamino-17-demethoxygeldanamycin | 17-N-allylamino-17-demethoxygeldanamycin | 17AAG | compound 4 [PMID:15978816]
Compound class:
Synthetic organic
Comment: 17-AAG inhibits the activity of hsp90. The compound is a derivative of the antibacterial geldanamycin.
As with other natural products, there is some ambiguity in the absolute stereochemistry annotated by different online resources. PubChem records an alternative isomer with a Z bond at position 12 which has CID 6505803. 17-AAG acts as a femtomolar inhibitor of Met tyrosine kinase receptor-dependent urokinase-plasminogen activation [5]. |
|
|||||||||||||||||||||||||||||||||||
| Selectivity at ligand targets | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||