|
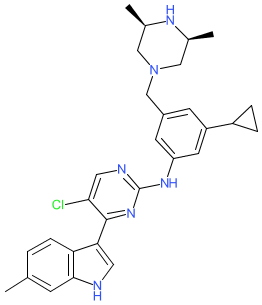
Synonyms: example 8 [US10870639B2] | HM-43239 | HM43239
Compound class:
Synthetic organic
Comment: HM43239 is an orally active, clinical stage compound that inhibits a number of kinases that are active in myeloid malignancies, including FLT3, SYK, mutant forms of cKIT, JAK1/2, and other kinases [1]. It is intended as a treament for patients with relapsed/refractory acute myeloid leukemia (AML). We matched its chemical structure to the INN 'tuspetinib' that was released in the WHO's proposed INN list 127 (21 July 2022).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| HM43239 retains inhibitory activity against the FLT3 internal tandem duplication (ITD) mutant (IC50 1.8 nM) [1]. It also inhibits VEGFR2 and SYK (% inhibition 100 and 95 respsctively; compound used at 100 nM for both kinase assays). |
| Selectivity at catalytic receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||