|
Compound class:
Synthetic organic
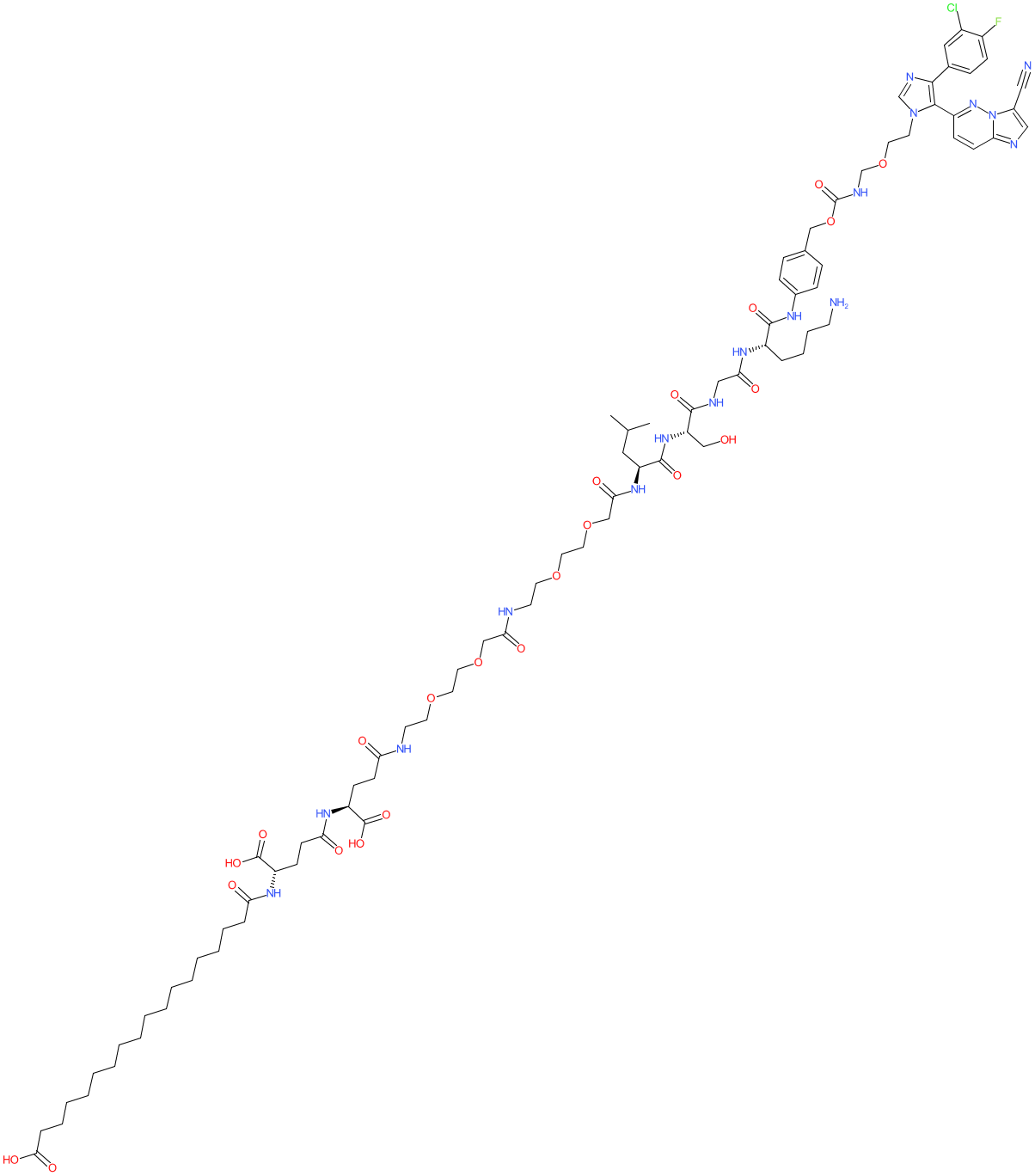
Comment: Compound 10 is a long-acting prodrug of the TGFβR inhibitor BMS-986260 [1]. It contains a matriptase peptide substrate domain, that is cleaved by the protease selectively in the tumour microenvironment. Compound 10 is sufficiently soluble to allow parenteral administration.
We generated the chemical structure shown here from the SMILES provided in the disclosure [1]. This prodrug approach is proposed to limit exposure of non-tumour tissue to the active parent molecule. |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Compound 10 potentiates the antitumour efficacy of anti-PD-1 checkpoint inhibitor monoclonal antibody treatment in the MC38 murine colon adenocarcinoma model (in which matriptase activity is upregulated). Antitumour efficacy of compound 10 was similar to that of the parent molecule in this model. |